Topic #13: Electron Transport Chain & OXPHOS
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Feeding ATP Production pt1
Oxidation of metabolic fuels —> preservation of their G in NADH & FADH2
A. Oxidation of NADH & FADH2 recycles these cofactors & release this G allowing it to be captured in ATP
1. ΔG° ́ NADH oxidation = -218 kJ/mol
2. ΔG° ́ FADH2 oxidation = -169 kJ/mol
3. ΔG° ́ ATP synthesis = 30.5 kJ/mol
Feeding ATP Production pt2
Both events will take place through the action of electron transport chain (ETC) & oxidative phosphorylation (OXPHOS)
A. Mitochondrion has about 70% thermodynamic efficiency in this process
1. Compared to car engine’s 30% efficiency
Recycling Cytosolic NADH
I. TCA cycle-produced NADH is already in mitochondrion & is sent directly into ETC
II. Glycolysis-produced NADH is in cytosol
A. Must be recycled to ensure glycolysis continues
B. IMM does not possess NADH transport protein, & NADH cannot directly cross it
1. e- from cytosolic NADH will enter mitochondrion via the action of 2 shuttle systems
Glycerol-3-Phosphate Shuttle
I. Major shuttle system in most tissues
II. Every cytosolic NADH entering by this shuttle system will generate ~ 1.5 ATP
Step 1: Glycolysis produces NADH in the cytosol, but NADH can't cross into the mitochondria directly.
Step 2: Cytosolic enzyme transfers electrons from NADH to DHAP, turning it into glycerol-3-phosphate and regenerating NAD⁺.
Step 3: Glycerol-3-phosphate carries the electrons to the edge of the mitochondrial inner membrane.
Step 4: A mitochondrial enzyme converts glycerol-3-phosphate back to DHAP, while passing the electrons to FAD (making FADH₂).
Step 5: FADH₂ feeds its electrons into the mitochondrial electron transport chain for ATP production
![<ul><li><p><span style="color: rgb(0, 0, 0);">I. Major shuttle system in most tissues</span></p></li><li><p><span style="color: rgb(0, 0, 0);">II. Every cytosolic NADH entering by this shuttle system will generate ~ 1.5 ATP</span></p></li><li><p><strong>Step 1:</strong> Glycolysis produces NADH in the cytosol, but NADH can't cross into the mitochondria directly.</p><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><strong>Step 2:</strong> Cytosolic enzyme transfers electrons from NADH to DHAP, turning it into glycerol-3-phosphate and regenerating NAD⁺.</p><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><strong>Step 3:</strong> Glycerol-3-phosphate carries the electrons to the edge of the mitochondrial inner membrane.</p><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><strong>Step 4:</strong> A mitochondrial enzyme converts glycerol-3-phosphate back to DHAP, while passing the electrons to FAD (making FADH₂).</p><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><strong>Step 5:</strong> FADH₂ feeds its electrons into the mitochondrial electron transport chain for ATP production</p></li><li><p></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/62173b29-3a00-444a-b571-861d47682221.png)
Malate-Aspartate Shuttle
I. This system occurs in the heart, liver, & kidneys
II. Every cytosolic NADH entering by this shuttle system will generate ~ 2.5 ATP 8
Step 1: Cytosol—Transfer Electrons to Malate
NADH from glycolysis transfers its electrons to oxaloacetate, making malate. NAD⁺ is regenerated so glycolysis keeps going.
Step 2: Malate Enters Mitochondria
Malate crosses into the mitochondria—carrying the electrons with it.
Step 3: Malate Delivers Electrons
Inside, malate turns back into oxaloacetate and regenerates NADH (now in the mitochondria). This NADH sends its electrons to the electron transport chain.
Step 4: Aspartate Keeps Shuttle Cycling
Oxaloacetate can’t leave, so it's converted into aspartate. Aspartate exits the mitochondria and is altered back to oxaloacetate in the cytosol, restarting the cycle.
know in cytocol trans e to go from malate, that malate goes into mito and delievers electros to etc asp comes out and thats what keep shuttle going (how e- in shuttle system)
![<p><span style="color: rgb(0, 0, 0);">I. This system occurs in the heart, liver, & kidneys<br>II. Every cytosolic NADH entering by this shuttle system will generate ~ 2.5 ATP 8</span></p><p><u><br></u><strong>Step 1: Cytosol—Transfer Electrons to Malate</strong><br>NADH from glycolysis transfers its electrons to oxaloacetate, making malate. NAD⁺ is regenerated so glycolysis keeps going.</p><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><strong>Step 2: Malate Enters Mitochondria</strong><br>Malate crosses into the mitochondria—carrying the electrons with it.</p><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><strong>Step 3: Malate Delivers Electrons</strong><br>Inside, malate turns back into oxaloacetate and regenerates NADH (now in the mitochondria). This NADH sends its electrons to the electron transport chain.</p><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2"><strong>Step 4: Aspartate Keeps Shuttle Cycling</strong><br>Oxaloacetate can’t leave, so it's converted into aspartate. Aspartate exits the mitochondria and is altered back to oxaloacetate in the cytosol, restarting the cycle.</p><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">know in cytocol trans e to go from malate, that malate goes into mito and delievers electros to etc asp comes out and thats what keep shuttle going (how e- in shuttle system)</p>](https://knowt-user-attachments.s3.amazonaws.com/1767cece-42e4-403e-908e-26febe223354.png)
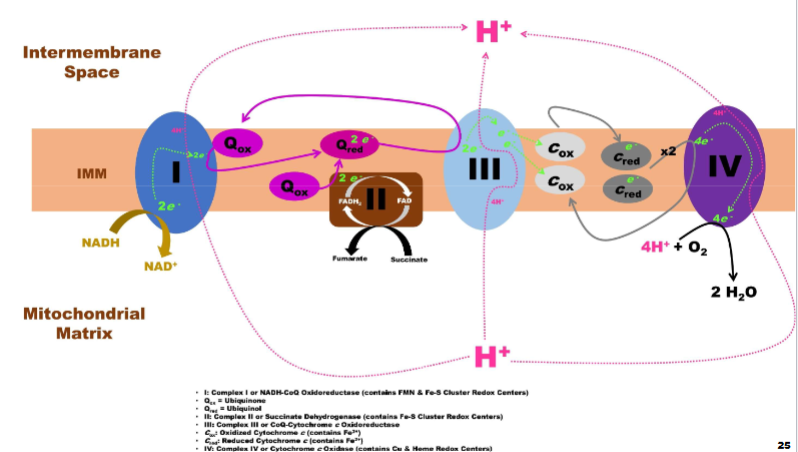
Electron Transport Chain aka Respiratory Chain pt1
def: series of 4 IMM-bound protein complexes & 2 small
mobile e- carriersA. Each component possesses a progressively greater affinity for e- (i.e., increasing E)
B. ETC receives e- from NADH & FADH2, passing them from 1 member to next until reaching final acceptor, O2
Electron Transport Chain aka Respiratory Chain pt2
As e- move through ETC (i.e., like electricity through a wire), provides G (energy) that drives pumping of H+ against its electrochemical gradient (hydrogen ion gradient)
A. H+ will be actively transported from mitochondrial matrix into intermembrane space
B. Accumulation of H+ against its electrochemical gradient represents a “build up” of G that can be used to do work (i.e., ATP production through OXPHOS)
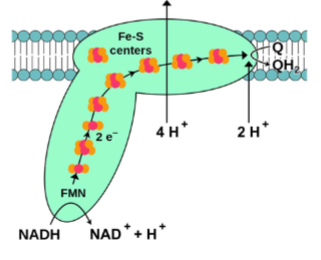
Complex I: NADH-CoQ Oxidoreductase pt1
Accepts 2e- from NADH, releasing NAD+ & H+ into mitochondrial matrix
A. 2e- are sequentially transferred (i.e., 1 at a time) through complex via multiple redox-active prosthetic groups (FMN & Fe-S clusters)
B. 2e- are delivered to ubiquinone (mobile, membrane-soluble molecule; also called coenzyme Q, CoQ, Q10) converting it into ubiquinol (reduced form)
Complex I: NADH-CoQ Oxidoreductase pt2
e- transfer provides G allowing for active transport of 4 H+ by complex from
mitochondrial matrix into intermembrane space (only when e- moving thru can u do this)
Leber Hereditary Optic Neuropathy (LHON)
Disease predominately occurring in young adults
A. Symptoms: sudden onset blindness due to degeneration of optic nerve
B. Cause: homoplasmic mutations (i.e., present in mtDNA of all cellular mitochondria) in Complex I (or II or IV)
1. Impair ETC e- flow & ATP synthesis
2. Detrimental to optic nerve due to its high energy demand & complete dependence on OXPHOS for ATP production (irreversible)
Leigh Syndrome
Caused by mutations in mitochondrial or nuclear genes encoding Complex I subunits
Usually appears in infants and young children
Early development is often normal, but neurological problems show up later
Results from damage to basal ganglia and other brain regions
Symptoms: ataxia, hypotonia, developmental regression, spongiosis, neuronal loss, astrocytosis, and increased capillary growth in the brai
Rotenone
Complex I inhibitor utilized as broad-spectrum
insecticide, piscicide, & pesticideA. Blocks e- transfer from Fe-S clusters to
ubiquinoneB. Occurs naturally in seeds & stems of several plants
Complex II: Succinate Dehydrogenase
Catalyzes the 6th step of the TCA cycle: converts succinate to fumarate, reducing FAD to FADH2
In the electron transport chain, oxidizes FADH2 back to FAD by transferring 2 H to iron-sulfur (FE-S) clusters and then to ubiquinone (forming ubiquinol)
Pumps no protons across the membrane, so FADH2 produces less ATP than NADH (big diff. form other complexes)
![<ul><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">Catalyzes the 6th step of the TCA cycle: converts succinate to fumarate, reducing FAD to FADH2</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">In the electron transport chain, oxidizes FADH2 back to FAD by transferring 2 H to iron-sulfur (FE-S) clusters and then to ubiquinone (forming ubiquinol)</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">Pumps no protons across the membrane, so FADH2 produces less ATP than NADH (big diff. form other complexes)</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/954a52f1-3f67-4850-ace9-11991a701d84.png)
Complex III: CoQ-Cytochrome c Oxidoreductase
Complex III receives electrons from 2 ubiquinol (QH2) molecules made by Complexes I and II
Transfers 2 e- through the complex and pumps 4 protons (H⁺) into the intermembrane space via the Q cycle
E- move through redox centers until 1 e- transferred to oxidized cytochrome c and reducing (Fe³⁺ to Fe²⁺)
Each Q cycle reduces 2 cytochrome c molecules, sending e- onward in the chain
as go thru complex 3 able to pump e-
![<ul><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">Complex III receives electrons from 2 ubiquinol (QH2) molecules made by Complexes I and II</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">Transfers 2 e- through the complex and pumps 4 protons (H⁺) into the intermembrane space via the Q cycle</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">E- move through redox centers until 1 e- transferred to <strong>oxidized cytochrome c</strong> and reducing (Fe³⁺ to Fe²⁺)</p></li><li><p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">Each Q cycle reduces 2 cytochrome c molecules, sending e- onward in the chain</p></li></ul><p>as go thru complex 3 able to pump e-</p>](https://knowt-user-attachments.s3.amazonaws.com/122ede5d-9d1f-4778-9a43-239d654761f8.png)
Antimycin A
Complex III inhibitor utilized as potent piscicide
A. Interferes with Q cycle (blocking movement of complex 3)
B. Naturally produced by Streptomyces bacteria
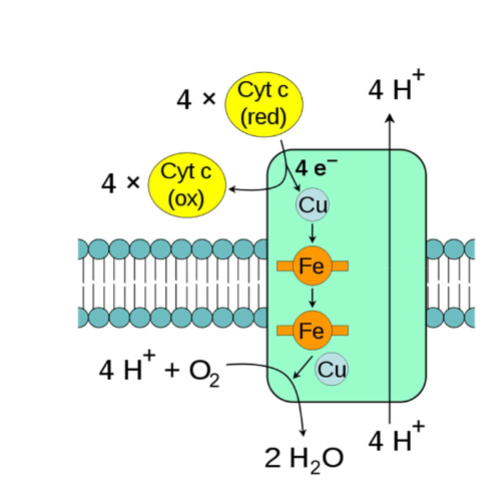
Complex IV: Cytochrome c Oxidase
I. Oxidizes 4 reduced cytochrome c (Fe2+) into 4 oxidized cytochrome c (Fe3+)
A. 4e- are passed to various redox centers (Cu complexes, Fe-containing cytochromes)
B. Final e- acceptor is O2, which is reduced by complex to form 2 H2O (called metabolic water)
1. Major reason human body requires O2
C. G generated by e- movement allows for pumping of 4 H+ into intermembrane space (2 per e- pair)
Cyanide
Cyanide (CN⁻) inhibits Complex IV of the electron transport chain, blocking ATP production
Acute poisoning with cyanide compounds (HCN, KCN) causes rapid death, used in chemical weapons and suicides
Cyanoglycosides containing cyanide are found in foods like almonds, stone fruit pits, cassava, soybeans, and others
Chronic cyanide poisoning from poorly processed bitter cassava causes a disease called Konzo
Oxidative Phosphorylation
I. Mitochondrial synthesis of ATP
II. Catalyzed by Complex V (ATP synthase)
III. ATP synthesis is driven by ETC, even though Complex V is physically distinct from other ETC complexes
A. Because G released by e- transport is conversed in a form usable by Complex V (called energy coupling or energy transduction)
components of etc