OCR A GCSE Chemistry: C2.2 Bonding
1/63
Earn XP
Description and Tags
Some From PMT + Some are added
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
What is the meaning of relative atomic mass?
The average mass of an atom of an element compared to 1/12th the mass of an atom of carbon-12.
What is the meaning of relative formula mass?
The weighted mean average masses of the formula units compared to 1/12th the mass of an atom of carbon-12.
What is the meaning of relative molecular mass?
The mean average mass of one molecule of an element or compound compared to 1/12th the mass of one atom of carbon-12.
How is relative formula mass calculated?
Add together the relative atomic masses of each of the elements in the chemical formula.
What is the relative formula mass of Ca(OH)2?
Calcium relative atomic mass = 40. Oxygen relative atomic mass = 16. Hydrogen relative atomic mass = 1. So formula mass = 40 + 2(16+1) = 74
What is the empirical formula?
The smallest whole number ratio of atoms of each element in a compound.
What is the molecular formula?
The formula that shows the actual number of atoms of each element in the compound.
Write the empirical formulae of CH4 and C4H10?
CH4 → CH4. CH4 is already in the smallest possible ratio. C4H10→ C2H5
What is the molecular and empirical formulae of the compound below?
Molecular formula: C2H4Br2. Empirical formula: CH2Br
What is an alloy?
A mixture of two or more metals.
What are metals and where are they found in the periodic table?
Metals are elements which react to form positive ions. They are found on the left side of the periodic table.
What are non-metals and where are they found in the periodic table?
Non-metals are elements which react to form negative ions. They are found towards the top right of the periodic table.
What are the general properties of metals?
Shiny, Good conductors, Dense, Malleable and ductile, High melting and boiling points
What are the general properties of non-metals?
Dull appearance, Poor conductors, Lower density than metals, Low melting and boiling points, Brittle
How are positive and negative ions formed?
Positive ions are formed when a metal loses an electron. Negative ions are formed when a non-metal gains an electron.
What is the chemical equation for the reaction between magnesium and oxygen?
2Mg + O2 → 2MgO. Mg forms the ion Mg2+ and oxygen forms the ion O2-.
How are elements arranged in the periodic table?
Elements are arranged in order of increasing atomic number so that elements in the same group (column) have similar properties.
Why do elements in the same column have similar properties?
They have the same number of outer shell electrons. This determines how they react.
What does the period (row) number tell you about all the elements in that row?
They all have the same number of shells of electrons. e.g. all elements in period 4 have 4 electron shells.
What does group (column) number tell you about all the elements in that group?
They all have the same number of outer electrons. e.g. all elements in group 2 have 2 electrons in their outer shell
Describe the difference between a covalent and ionic bond
A covalent bond forms when two non-metals share a pair of electrons. An ionic bond forms between a positive metal ion and negative non-metal ion. Covalent bonds only occur between non-metals and they do not involve any charged particles while ionic bonds include both metal and non-metal ions.
Describe the bonding in an ionic compound
Ionic bonds form between positive metal ions and negative non-metal ions. Ionic compounds are held together by the electrostatic attraction between these oppositely charged ions.
Why do ionic compounds have high melting and boiling points?
The strong electrostatic forces of attraction between oppositely charged ions require a lot of energy to overcome.
When do ionic compounds conduct electricity? Why?
Ionic compounds conduct electricity when molten or aqueous because the ions are free to move to carry charge. When solid, the ions are fixed in the ionic lattice so don't conduct electricity.
Describe the bonding in simple molecules
Covalent bonds, formed when two non-metals share a pair of electrons.
Why do simple molecules have low boiling points despite containing strong covalent bonds?
To change state, simple molecules need to overcome the intermolecular forces, not the covalent bonds. Simple molecules are held together by weak intermolecular forces which require little energy to overcome.
Why are simple molecules unable to conduct electricity?
They have no overall charge.
How and why do boiling points of simple molecules change as the size of the molecules increases?
As the size of the molecule increases, the strength of the intermolecular forces also increases. Larger simple molecules have higher boiling points as more energy is required to overcome the intermolecular forces.
Describe the bonding in giant covalent structures
Many strong covalent bonds (shared pair of electrons).
Why do giant covalent structures have very high melting points?
All of the atoms in the structure are covalently bonded to other atoms. These strong covalent bonds must be broken to melt the substance which requires a lot of energy meaning the melting point is very high.
What type of bonds are found in polymers?
Covalent bonds
Why are polymers solids at room temperature?
Polymers are simple molecules so their melting point depends on the strength of the intermolecular forces. As the molecules are very large, the intermolecular forces are strong so require a lot of energy to overcome in order to melt the polymer.
Describe the structure and bonding in metals
Metallic bonding. Giant structure with positive metal ions held in a sea of delocalised electrons.
Why are metals able to conduct electricity?
The delocalised electrons are free to move throughout the structure so can carry charge through the metal.
Why are metals typically very malleable?
The atoms in metals are arranged in uniform rows that can easily slide over one another. This allows metals to be bent and shaped.
Why do metals have relatively high melting points?
They have very strong metallic bonding. A lot of energy is required to overcome the electrostatic attraction between the positive ions and negative electrons.
Draw a dot and cross diagram for methane, CH4
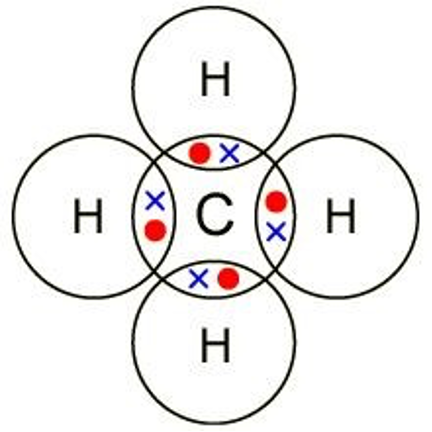
Draw a dot and cross diagram for NaCl
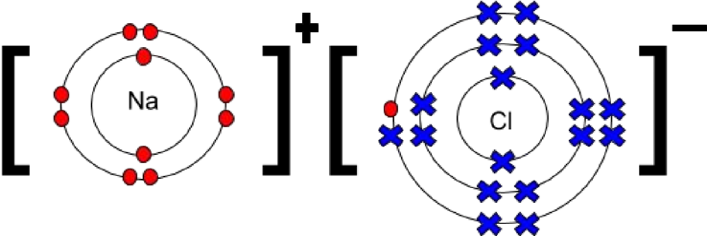
What is a limitation of dot and cross diagrams?
Don’t show the 3D arrangement of molecules.
What is a benefit of using ball and stick models to represent molecules? In what way are they limited?
They show the 3D shape and how atoms are bonded. - They don’t model electrons.
What is the highest electron configuration when looking at the first three shells?
2, 8, 8
What is the most desirable electron configuration?
All atoms want to have a full outer shell so this would be 8 electrons in the outer shell (or 2 if the atom only has one shell).
Why are the noble gases (group 0) very unreactive?
They have very stable electron configurations due to their full outer shell of electrons. This means they don’t want to lose or gain electrons so are unreactive.
Why might an element with the electron arrangement of 2, 8, 1 be very reactive?
It’s very reactive because it can lose an electron (becoming a positive ion) to obtain the stable configuration 2,8.
How did Mendeleev order his early periodic table?
In order of increasing atomic mass. In some places, the order was changed slightly so that elements of similar properties would be grouped in the same column.
Why did Mendeleev leave gaps in his periodic table?
For undiscovered elements. He used elements around these gaps to predict properties of the missing elements.
Some elements in Mendeleev’s table did not fit with the expected properties. How has this been modified in the modern periodic table?
The elements are now ordered by increasing atomic number rather than increasing atomic mass.
How many covalent bonds can carbon form?
Four
Describe the structure of graphite
Each carbon atom bonded to 3 other carbon atoms. Layers of hexagonal rings of carbon atoms. One delocalised electron per carbon atom.
Describe the properties of graphite
Soft/ slippery because the weak intermolecular forces between layers allow the layers to slide over one another. Electrical conductor because it contains delocalised electrons which are free to carry charge.
Describe the structure of diamond
Each carbon atom is covalently bonded to four other carbon atoms. No charged particles.
Describe the properties of diamond
Very hard and very high melting point due to strong covalent bonds. Doesn’t conduct electricity because there are no charged particles.
What is a fullerene?
A molecule made up of carbon atoms, shaped like a closed tube or hollow ball.
Name two fullerenes
Graphene, C60 (buckminsterfullerene)
What are the properties and uses of fullerenes?
They have a large surface area so are useful for trapping catalysts onto their surfaces. Hollow structure makes them useful for capturing substances by forming around the target molecule. Useful for targeted drug delivery systems.
Why is graphene useful in electronics?
It is extremely strong. It has free electrons so can conduct electricity. It’s only one atom thick (single layer of graphite).
Energy is transferred to the surroundings during which changes of state?
Condensing, Freezing
Energy is transferred to a substance during which changes of state?
Evaporating, Melting
Substance A melts at -200oC and boils at -183oC. What state is A at -174oC?
Liquid
SubSTANCE B melts at -5oC and boils at 23oC. What state is A at -7oC?
Solid
Do individual atoms have the same physical properties of the substance that contains them? Explain your answer
No, physical properties of a substance depend on the bonds it contains as well as the strength and arrangement of these bonds.
How can you calculate the surface area to volume ratio? (Chemistry only)
Surface area to volume ratio = Surface area ÷ Vol
What can covalent bonding be described as (in terms of electrostatic attraction)?
An electrostatic attraction between shared pairs of electrons.
What can metallic bonding be described as (in terms of electrostatic attraction)?
An electrostatic attraction between delocalized electrons and positively charged metal ions.