Preparation and properties of Ethene
1/20
Earn XP
Description and Tags
CALCULATIONS. Also watch experiment video before studying these flashcards to recap.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Draw a labelled diagram of a suitable arrangement of apparatus and of chemicals for the preparation and collection over water of samples of pure ethene gas
Horizontal test tube with glass wool soaked in ethanol and deliver tube shown //
heat source under catalyst near centre of test tube //
collection of ethene over water
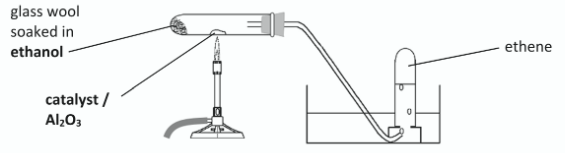
When carrying out this preparation, state one way of avoiding
i) a suck-back of cold water into the reaction vessel,
ii) a fire
i) loosen stopper /
remove tube from water to avoid partial vacuum /
do not cool with tube in water
Some ethene was bubbled through a reagent as shown in the diagram on the right.
What colour change is observed when the reagent used is
i) dilute acidified KMnO4,
ii) bromine water?
i) purple to colourless
ii) brown to colourless
iii) Name the organic reaction type that occurs in the case of the bromine reagent
Addition
iv) Identify an organic product of the reaction between ethene and the bromine solution
1,2-dibromoethane
Identify the catalyst and describe it’s appearance at the beginning of the preparation
Alumina
White / powder / solid
Explain clearly why a risk of hot glassware shattering, due to rapid cooling, is associated with this preparation.
How can this risk be minimised?
Suck-back possible / cold water enters hot glassware
Remove tube from water before reducing heat /
loosen stopper before reducing heat /
remove tube from water to avoid partial vacuum creation /
loosen stopper to avoid partial vacuum creation
Combustion tests were carried out on samples of ethene and ethyne.
Compare the observations made in the two tests
Ethyne flame sootier/smokier/more luminous than ethene’s
Ethene flame cleaner/less luminous than ethyne’s
Ethyne sooty / ethene clean
Write a balanced equation for the complete combustion of ethyne in oxygen
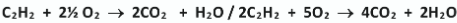
Name a reagent used to test the gases ethene and ethyne for unsaturation
Bromine solution (water)
A sample of ethene gas was supplied in a stoppered test tube. Describe fully how the gas could have been shown to be unsaturated
Bromine soln //
brown //
to colourless
At what stage in the procedure is a ‘suck-back’ most likely to occur?
When heat is removed / at the end
Give one possible consequence of ‘suck-back’ occurring
Cold water sucked into hot test tube / test tube cracks / fire / explosion / injury due to broken glass
How could a ‘suck-back’ be avoided?
Remove delivery tube from water before removing heat /
loosen stopper before removing heat
Write a balanced equation for the preparation of ethene from ethanol
C2H5OH → C2H4 + H2O
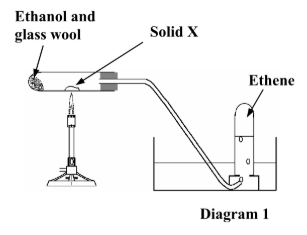
Give the name or chemical formula of the solid X used in the preparation of ethene.
What is the colour of this solid?
Alumina / aluminium oxide / Al2O3
White
Write a balanced equation for the reaction involved in the preparation of ethene.
What term describes this type of reaction?
C2H5OH → C2H4 + H2O
Elimination
State three precautions that should be observed when carrying out the preparation of ethene by this method
Keep gas from flame / air-tight stopper / secure assembly //
safety screen / glasses / protective clothing / tie hair back //
before heat removed take tube from water //
use tongs / gloves //
avoid inhaling glass wool / wear mask
What is the function of the glass wool in the preparation of ethene?
Keeps (soaks up) ethanol at the end of test tube / avoid wetting the aluminium oxide
Describe the flame that would be observed when a combustion test is carried out on a sample of ethene gas.
Yellow / luminous
Write a balanced chemical equation for the combustion of ethene in excess oxygen
