Pauli Exclusion Principle
0.0(0)
Card Sorting
1/6
Earn XP
Description and Tags
Last updated 3:39 AM on 10/6/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
1
New cards
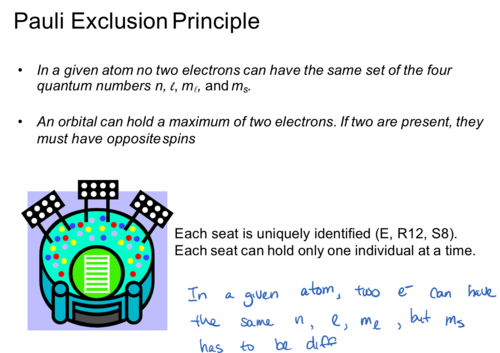
Pauli Exclusion Principle
In a given atom no two electrons can have the same set of the four
quantum numbers n, l, m, and ms.
An orbital can hold a maximum of two electrons. If two are present, they
must have opposite spins
In a given atom no two electrons can have the same set of the four
quantum numbers n, l, m, and ms.
An orbital can hold a maximum of two electrons. If two are present, they
must have opposite spins
allow
2
New cards
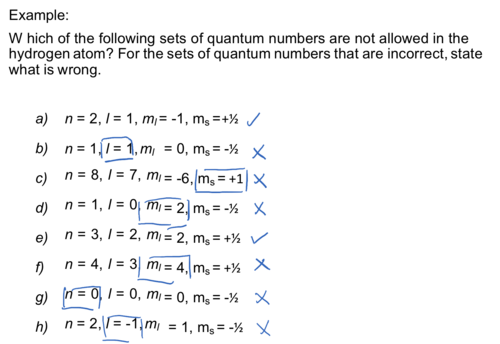
Example:
W hich of the following sets of quantum numbers are not allowed in the hydrogen atom? For the sets of quantum numbers that are incorrect, state what is wrong.
W hich of the following sets of quantum numbers are not allowed in the hydrogen atom? For the sets of quantum numbers that are incorrect, state what is wrong.
allow
3
New cards
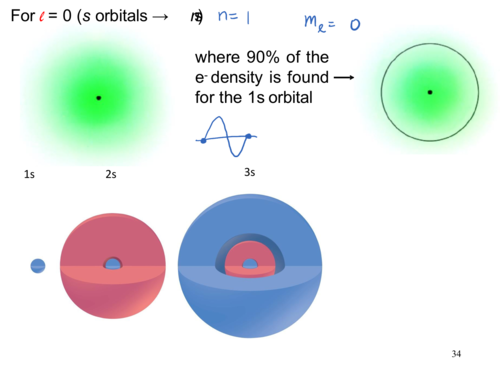
For l = 0 (s orbitals→ns)
yes
4
New cards
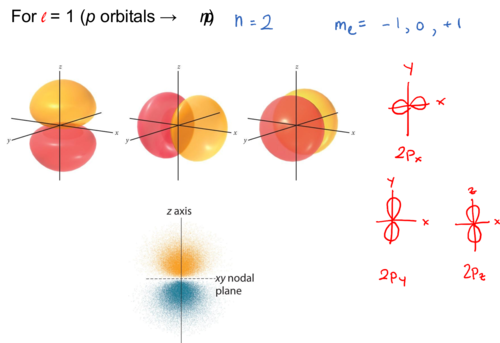
For l = 1 (p orbitals → np)
5
New cards
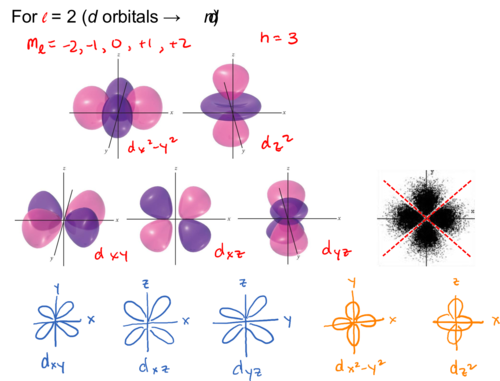
For l = 2 (d orbitals → nd)
6
New cards
Example:
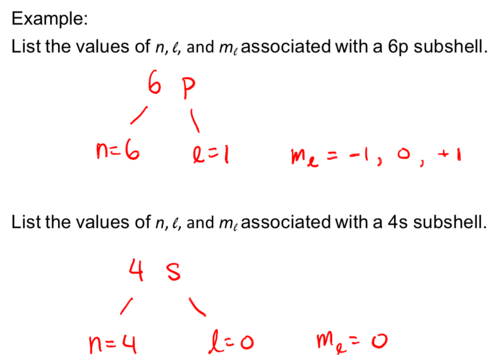
List the values of n, l, and ml associated with a 6p subshell.
List the values of n, l, and ml associated with a 4s subshell.
List the values of n, l, and ml associated with a 6p subshell.
List the values of n, l, and ml associated with a 4s subshell.
7
New cards
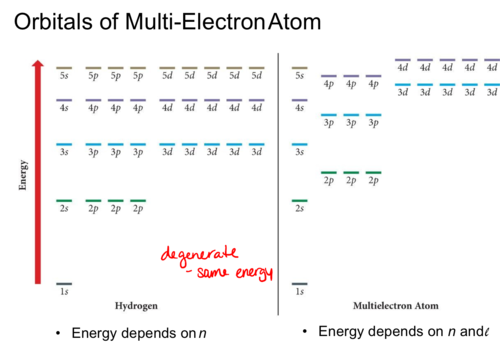
Orbitals of Multi-Electron Atom