A Level CIE Biology: 12 Energy and Respiration
1/169
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
170 Terms
what does work in a living organism require?
energy and usable carbon compounds
four types of work
transporting substances across membranes
anabolic reactions
movement
maintaining body temperature
two examples of transporting substances across membranes
active transport using the sodium-potassium pump in cell membranes
exocytosis of digested bacteria from wbcs
two examples of anabolic reactions
synthesis of DNA from nucleotides
synthesis of proteins from aas
two examples of movement
cellular movement of chromosomes via the spindle
mechanical contraction of muscles
example of maintaining body temperature
occurs only in mammals and birds
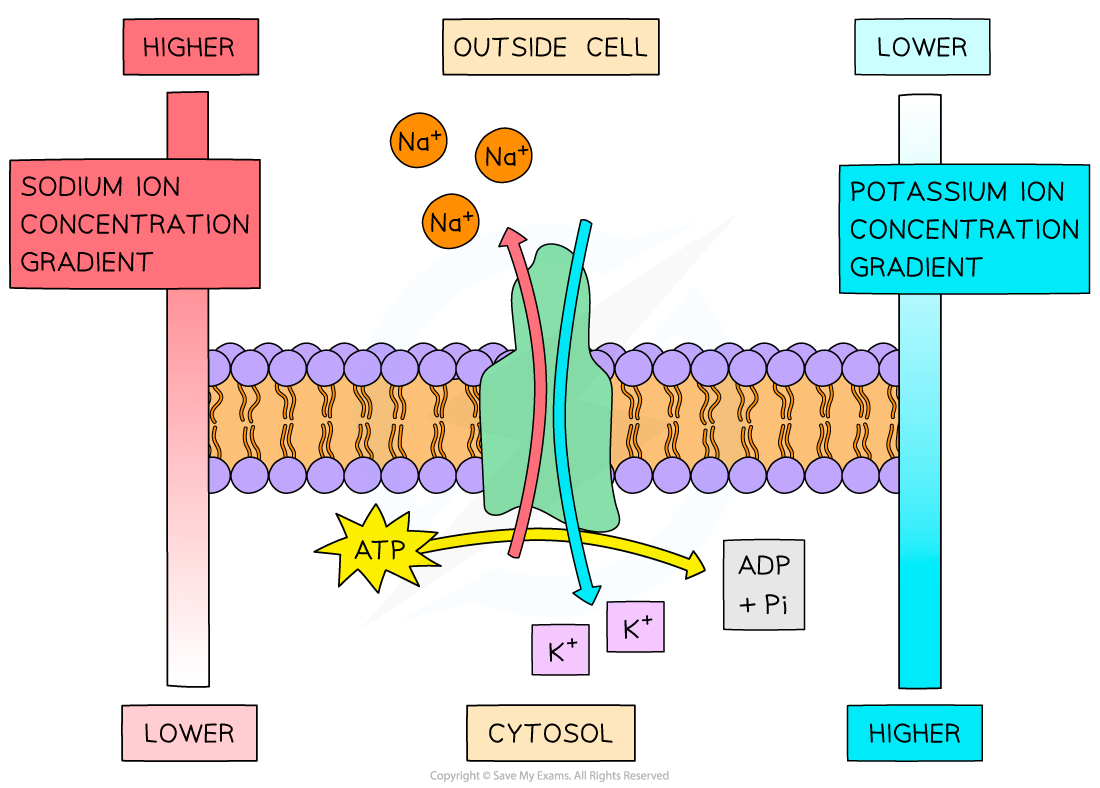
description of energy in active transport diagram
as the metal ions are both moving against their concentration gradient, they cannot move by simple diffusion. require carrier protein and ATP
what is the primary source of energy for nearly all organisms
the sun
the reactions of photosynthesis store energy in ______ ________
organic molecules
light energy from the sun is transformed into…
chemical potential energy in the synthesis of carbohydrates
what happens to the carbs formed
used in synthesis of ATP from their breakdown or are combined and modified to form al the usable organic molecules that are essential for all metabolic processes within the plant
photosynthesis is carried out by…
the first organism in a food chain, such as plants and other small organisms such as blue-green algae
what is respiration in all living cells…
releases energy from the breakdown of organic molecules
respiration involves…
transfer of chemical potential energy from nutrient molecules e.g. carbs, fats and proteins into a usable energy form through the synthesis of ATP that can be used for work within an organism1
glucose + oxygen →
carbon dioxide + water [+ energy]
C6H1206 + 6 O2 →
6 CO2 + 6 H20 (+ 2870kJ)
autotrophs:
organisms that are able to synthesize their own usable carbon compounds from carbon dioxide in the atmosphere through photosynthesis
heterotrophs:
require supply of pre-made usable carbon compounds which they get from their food
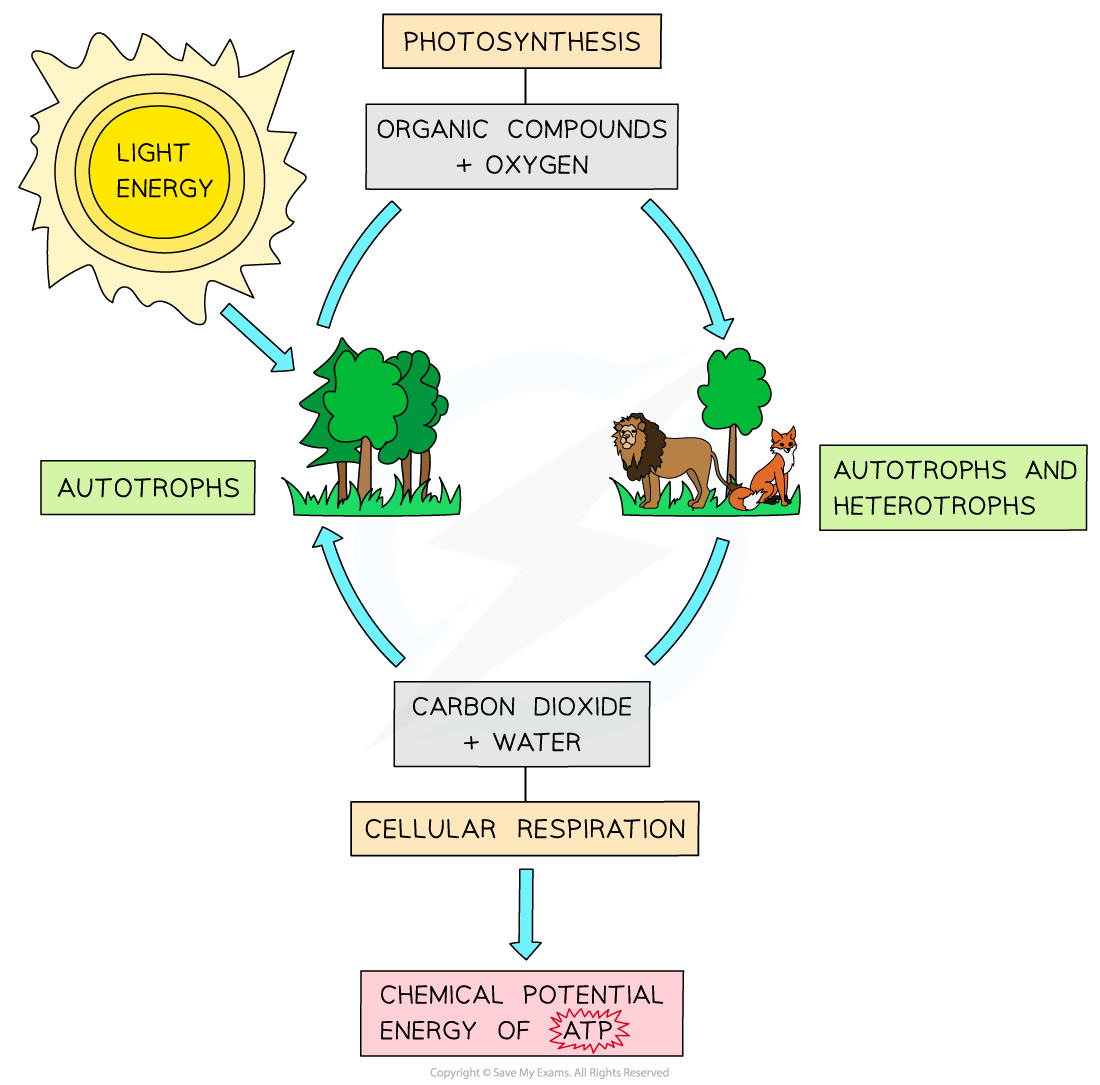
description of reactions of photosynthesis and respiration
transfer of energy and materials between autotrophs and heterotrophs through the processes of photosynthesis and respiration
laws of thermodynamics:
energy cannot be created or destroyed, it is transformed
adenosine triphosphate [atp]
the molecule that energy released during respiration is transferred
atp:
small, soluble molecule that provides a short-term store of chemical energy that cells can use to do work
atp is vital in…
linking energy-required and energy-yielding reactions
why is atp described as a universal energy currency
universal: used in all organisms
currency: can be used for diff purposes and reused countless times
three reasons atp as an energy currency is beneficial:
hydrolysis of atp can be carried out quickly and easily when energy is required within cell by action of just atpase
useful qt. of energy released from hydrolysis of one atp mol so reduces waste and gives cell control over processes
atp relatively stable at cellular pH levels
atp is a _____ nucleotide
phosphorylated
atp is made up of what three things
ribose sugar
adenine base
three phosphate groups
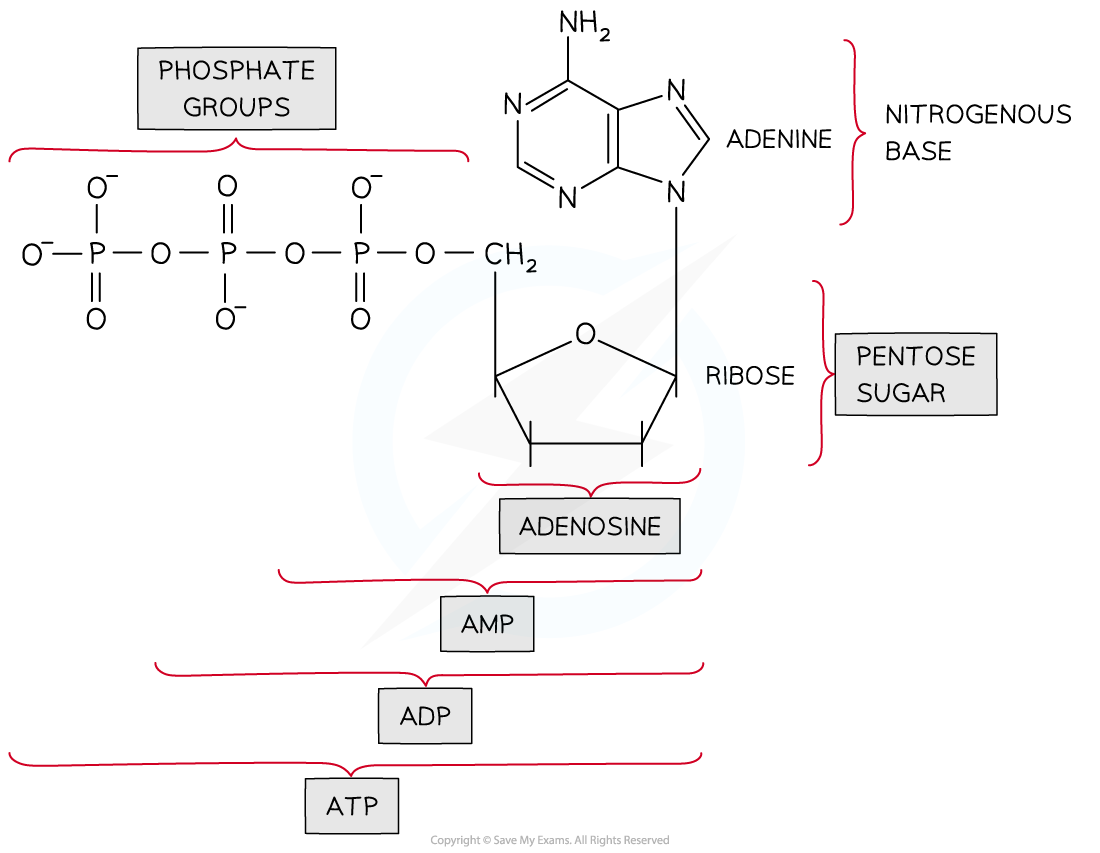
what is produced when atp is hydrolysed
adp and phosphate
as adp is formed what is released and what is that used for
free energy, can be used for processes within cells e.g. dna synthesis
removal of ___ phosphate group releases ____ kJmol-1 of energy, forming ___
one phosphate group from ATP releases approximately 30.5 kJ mol -1 of energy, forming ADP
second phosphate group from ADP also releases approximately 30.5 kJ mol-1 of energy, forming AMP
third and final phosphate group from AMP releases 14.2 kJ mol-1 of energy, forming adenosine
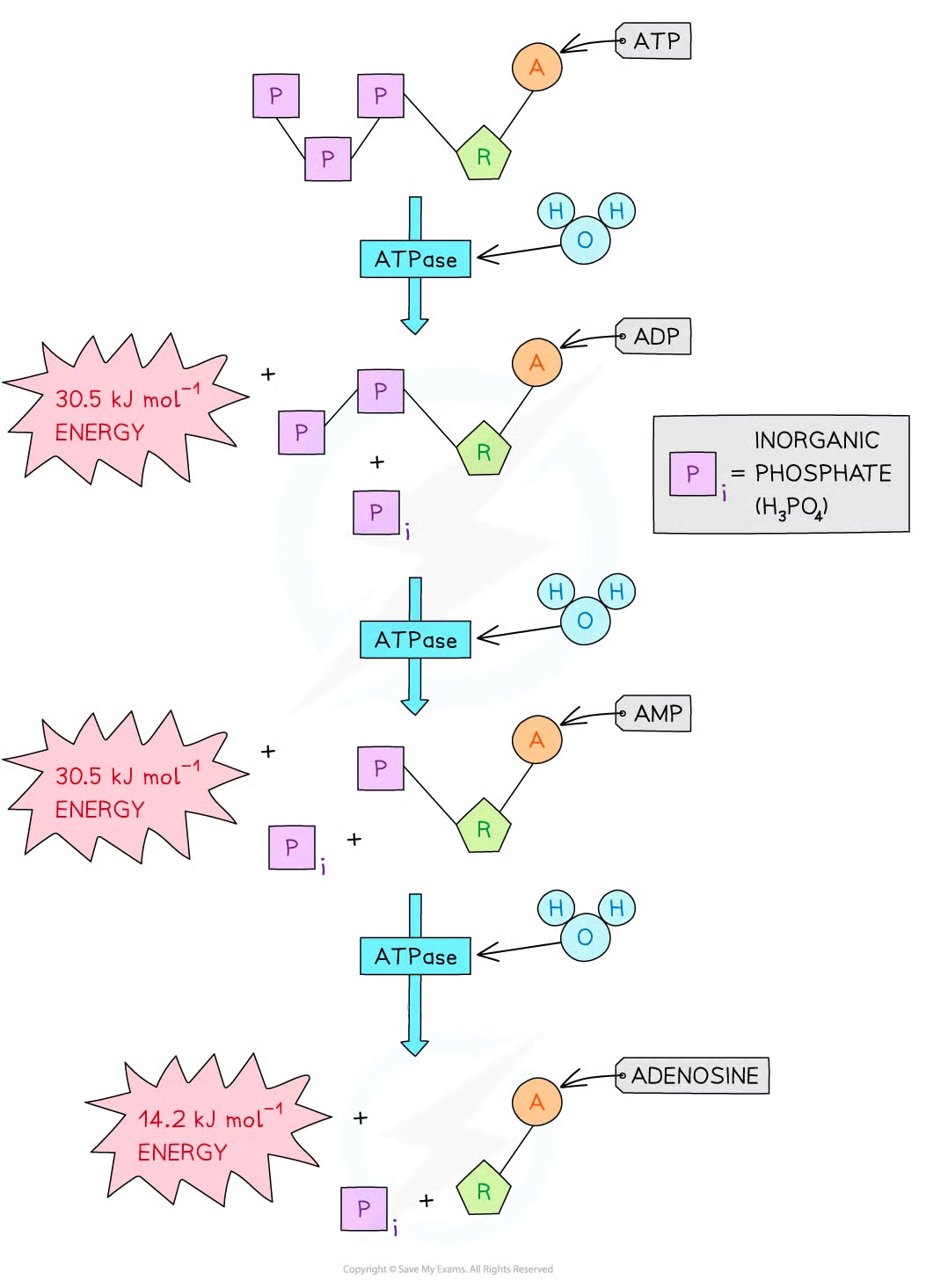
six features of atp
releases a small but sufficient amount of energy (75.2 kJ mol-1 from the complete hydrolysis of ATP)
exists as stable mol
can be recycled
quick and easy hydrolysis
soluble and moves easily in cell
forms phosphorylated intermediates
benefit of releasing small but sufficient energy (75.2 kJ mol-1 from the complete hydrolysis of ATP)
enough energy to drive important met. reactions while keeping energy wastage low
benefit of atp’s stability
doesn’t breakdown unless atpase/catalyst is present so no energy waste
benefit of atp being recyclable
breakdown of atp is reversible and can be reformed from adp and pi meaning same mol can be used elsewhere in cell for diff reactions
benefit of atps quick and easy hydrolysis
cells can respond to sudden increase in energy demand
benefit of atps solubility and easy movement
can transport energy to diff areas of cell
benefit of atp forming phosphorylated intermediates
can make metabolites more reactive and lower activation energy needed for reaction
energy:
capacity/power to do work
why must cells make atp as and when they need it
organisms cannot build up large stores of atp and it rarely passes thru the csm. humans use 50 kg of ATP in a day but only have a maximum of ~ 200g of ATP in their body at any given time
atp is formed when adp is combined w/ inorganic phosphate [pi] group. what two things happen during this reaction
energy requiring
water released as waste product [atp synthesis = condensation reaction]
![<ul><li><p>energy requiring</p></li><li><p>water released as waste product [atp synthesis = condensation reaction]</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/48a01657-cfb4-416e-866b-72d4e590be7f.png)
when is atp made
during respiration or photosynthesis
two ways atp can be made
substrate-linked phosphorylation
chemiosmosis
how does SLP work
atp is formed by transferring phosphate directly from substrate mol to ADP
SLP equation
ADP + Pi → ATP
where is energy required in SLP from
provided directly by another chemical reaction
when and where does SLP occur
in glycolysis. cell cytoplasm and mitochondria matrix
what amt of atp synth does slp account for during aerobic resp
small amt ~ 4 / 6 ATP per glucose molecule
what does chemiosmosis involve
a proton/hydrogen ion gradient across a membrane
where does chemiosmosis take place
across inner memb of mitoch and thylakoid memb of chloroplasts
how is proton concentration gradient established in chemiosmosis
electron transport chain where high energy e- move from carrier to carrier releasing energy that is used to pump protons up a conc grad across the inner memb into the intermembrane space
chemiosmosis: protons are pumped from___ conc in __________ to ___ conc in _______
low, inner mitoch matrix, high, intermembrane space
chemiosmosis: when is energy released
when protons move down conc grad into matrix
chemiosmosis: how is atp phosphorylated
protons move through atp synthase complex with released energy driving phosphorylation
chemiosmosis: why is water formed
oxygen acts as final e- and proton acceptor to form water
how much atp is synthesised via chemiosmosis during resp
most : ~ 32 / 34 ATP per glucose molecule
SLP proces
phosphate of a substrate mol is transferred directly from adp to atp and uses energy provided directly by another chem reaction.
chemiosmosis process
energy release by movement of h ions down conc grad is used to synthesize atp via enzyme atp synthase. oxygen acts as final hydrogen/electron acceptor
______ is the principal respiratory substrate for aerobic resp in most cells
glucose
how does the cell continue respiration when glucose supply is used up
by using other substrates such as carbs, lipids, proteins
when are amino acids respired aerobically and why
only when all other substrates have been exhausted because they often have essential functions elsewhere in the cell and are required to make proteins with structural e.g. cytoskeleton and functional e.g. enzymatic roles.
respiratory substrate [carb, lipid, protein] and energy value/kJg-one
carb - 15.8
lipid - 39.4
protein - 17.0
why are the energy value of substratees different
because they have different molecular compositions and number of hydrogen atoms that become available when substrate mols are broken down
what happens to hydrogen during respiration
sub mols broken down and H atoms become available
H carrier mols called NAD and FAD pick them up/become reduced and transfer them to inner mitoch membrane
reduced NAD and FAD release H atoms which split into protons and electrons
protons are pumped across inner mitoch memb into intermembrane space forming proton/chemiosmotic gradient
proton grad in chemiosmosis used to make atp
after protons have flowed back into matrix of mitoch via atp synthase they are oxidised to form water
mols w/ higher hydrogen content will result in…
greater p+ gradient across mitoch memb which allows for formation of more ATP via chemiosmosis e.g. fatty acids release H when lipid broken down

respiratory content [rq]:
ratio of carbon dioxide mols produced to oxygen mols taken in during resp

rq:
co2/o2

why do carbs, lipids and proteins have diff rq values
because no. C-H bonds differs in each type of bio molecule
higher no. C-H bonds means that…
more H atoms used to create p+ gradient meaning that more ATP mols can be produced by chemiosmosis so more oxygen is required to break down the mol [in last step of oxidative phosphorylation to form water]

when glucose is respired aerobically…
equal volumes of carbon dioxide are produced and oxygen take in meaning it has rq value of one
carb, protein, lipid typical rq value
carb - 1.0
protein -0.8 - 0.9
lipid - 0.7
mammalian muscle cells and plant tissue cells use what anaerobic resp
lactate fermentation, ethanol fermentation
can the rq for anaerobic respiration in animals and yeast be calculated
cannot be calculate because no oxygen used and no carbon dioxide produced during lactate fermentation and for yeast, the rq tends towards infinity as no oxygen is used but carbon dioxide is produced
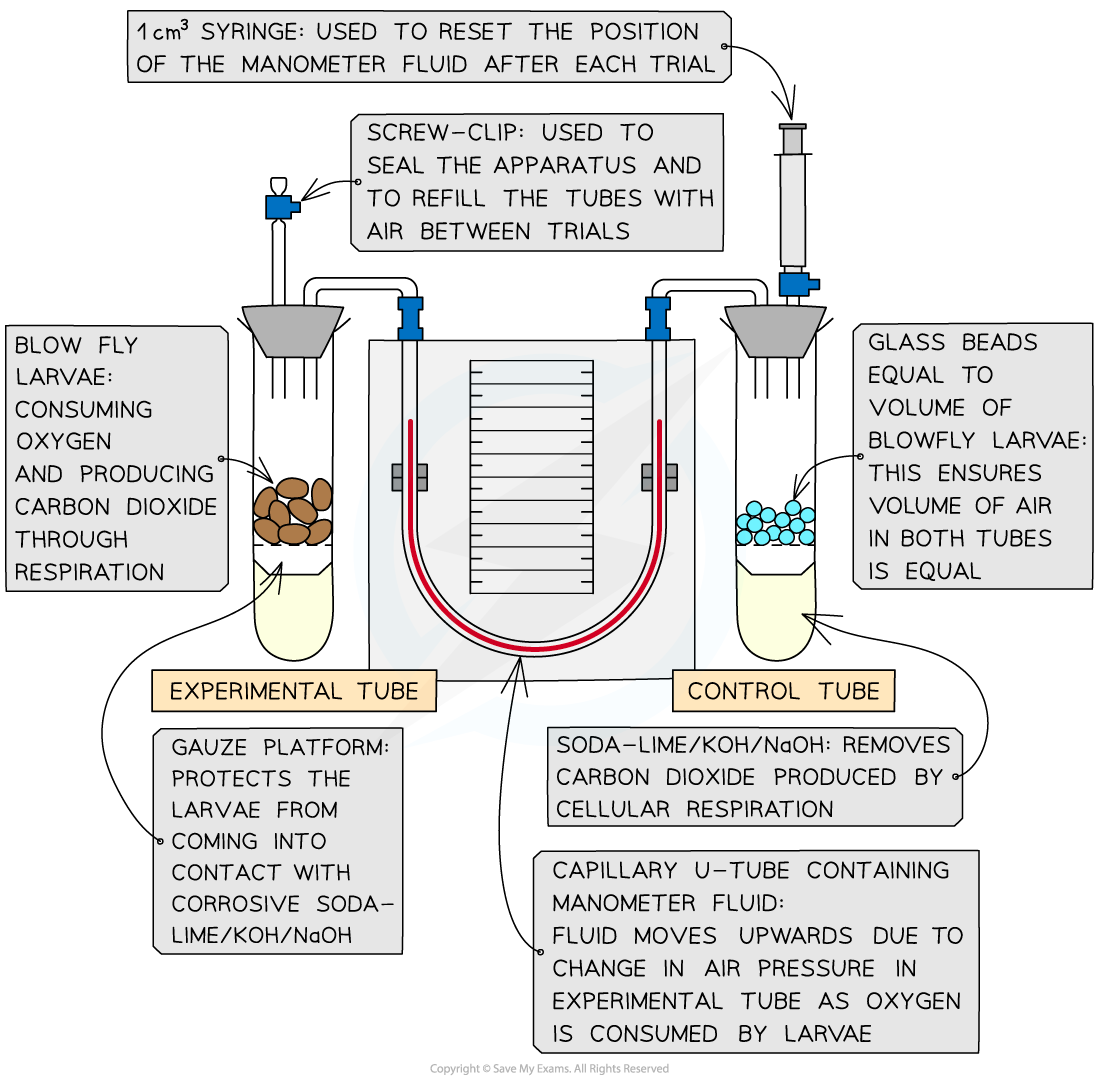
respirometers use
measure and investigate the rate of oxygen consumption during respiration in organisms and calculate rq.
what are respirometers usually involved with
experiments with germinating seeds or invertebrates
equation for calculating change in gas volume =
volume of oxygen [cm3 min-1] can be worked out using the diameter of the capillary tube r (cm) and the distance moved by the manometer fluid h (cm) in a minute using the formula: πr2h
what is the method of using a respirometer to determine the rq
measure oxygen consumption, set up respirometer and run experiment w/ soda-lime present in both tubes
use 1manometer to calc change in gas volume in given time x cm3 min-1
always read from side of the u-tube manometer closest to respiring organism [left]
reset apparatus by allowing air to re-enter tubes via screw cap and reset manometer fluid w/ syringe
run experiment again and remove soda-lime from both tubes and use manometer reading to calc change in gas volume in given time y cm3 min-1
what do x and y give in respirometer experiment and how to use them to calculate
x : volume of oxygen consumed by respiration within given time
y: volume of oxygen consumed by respiration within a give time - volume of carbon dioxide produced within given time
[x-y] = volume of carbon dioxide given off by organisms. rq=x-y/x
what three things could it mean when the rq value changes
substrate being respired has changed
or that cells are using a mixture of substrates in respiration
overfeeding [more than one = excessive carb/calorie intake, less than .seven = underfeeding]
under normal cellular conditions what is the order that substrates are used in respiration
carb → lipid → protein
what is mitochondria [shape, size, function]
rod-shaped organelles 0.5 - 1.0 µm in diameter
site of aerobic resp in euk cells
synthesises atp during last stage of resp = oxidative phosphorylation
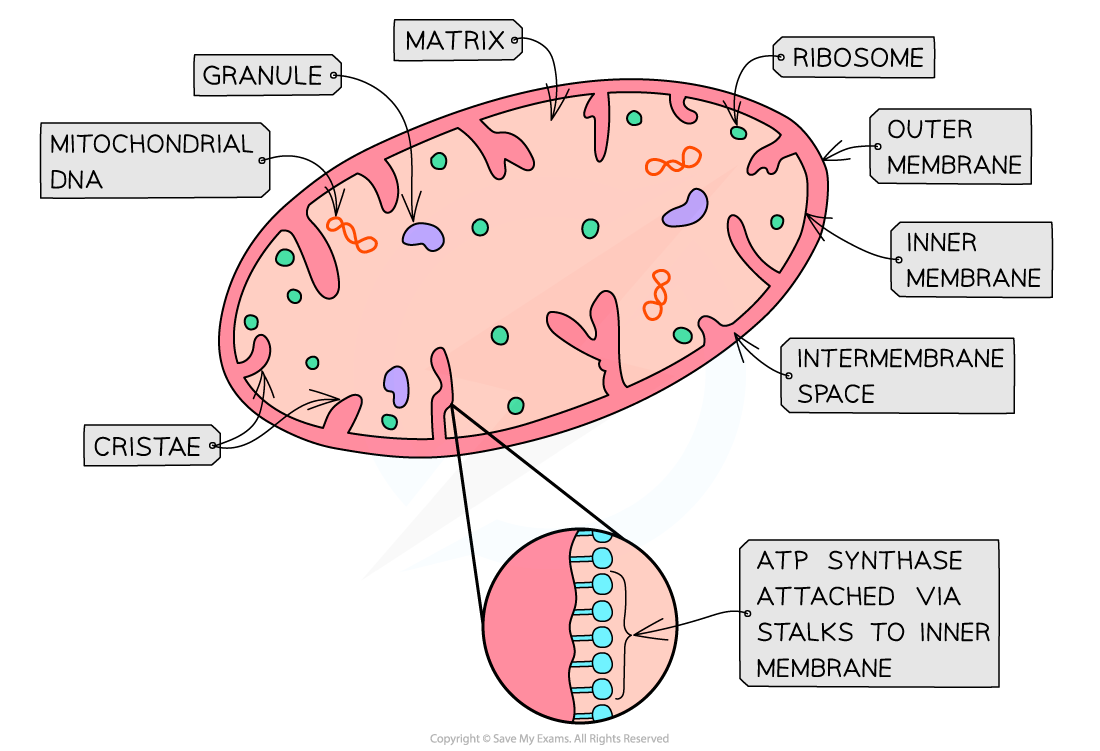
what does oxidative phosphorylation rely on
proteins that make up electron transport chain and atp synthase enzyme
![<p>mitochondria structure [four parts]</p>](https://knowt-user-attachments.s3.amazonaws.com/39625180-a579-4459-9f69-2039c5da7b06.png)
mitochondria structure [four parts]
two phospholipid membranes
outer membrane
inner membrane
intermembrane space
outer membrane is: [two]
smooth
permeable to several small molecules
inner membrane is [four]: [pic of crristae]
folded cristae
less permeable
site of e- transport transport chain used in oxidative phosphorylation
location of atp synthase used in oxidative phosphorylation

intermembrane space two features
low pH due to high conc. of protons
conc grad across inner memb is formed during oxidative phosphorylation and is essential for atp synthesis
matrix two features: [pic of mitoch]
aqueous solution within inner membranes of mitochondrion
contains ribosomes, enzymes and circular mitoch dna necessary for mitoch to function
the structure of mitoch makes them…
well adapted to their function
mitoch large sa reason + benefit
bc presence of cristae [inner folds] which enables membrane to hold many e- transport chain proteins and atp synthase enz
why can more active cells have a larger mitoch w/ longer and more tightly packed cristae
to enable synthesis of more atp bc larger sa
no. mitoch in each cell varies depending on…
cell activity e.g. muscle cells more active have more mitoch per cell than fat cells due to diff levels of metab. activity in those cell types = diff levels of demand for atp
four stages of aerobic respiration
glycolysis - takes place in cell cytoplasm
link reaction - takes place in matrix of mitoch
krebs cycle - takes place in matrix of mitoch
oxidative phosphorylation - occurs in inner membrane of mitoch
stages of respiration description + location
glycolysis : phosphorylation and splitting of glucose in cell cytoplasm
link reaction : decarboxylation and dehydrogenation of pyruvate in mitoch matrix
krebs cycle : cyclical pathway w/ enz-controlled reaction in mitoch matrix
oxidative phosphorylation : production of atp through oxidation of H atoms in inner memb of mitoch
when does glycolysis take place and involve:
cytoplasm of cell
trapping glucose in cell by phosphorylating molecule
splitting glucose mol into two
what does glycolysis result in
production of:
2 pyruvate (3C) molecules
Net gain 2 ATP
2 reduced NAD
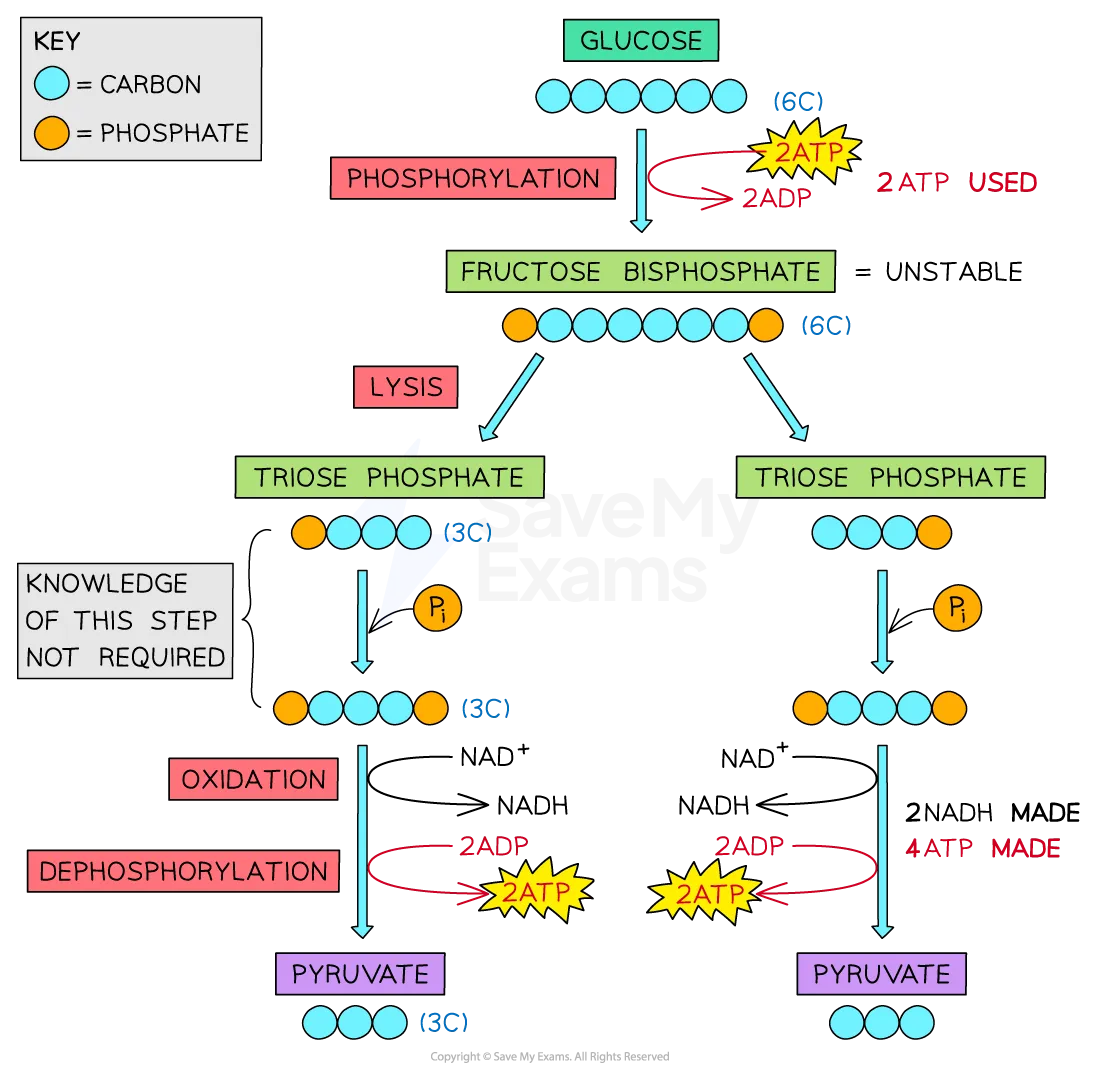
phosphorylation:
glucose (6C) is phosphorylated by 2 ATP to form fructose bisphosphate (6C)
Glucose + 2ATP → Fructose bisphosphate
lysis:
fructose bisphosphate (6C) splits into two molecules of triose phosphate (3C)
Fructose bisphosphate → 2 Triose phosphate
oxidation:
hydrogen is removed from each molecule of triose phosphate and transferred to coenzyme NAD to form 2 reduced NAD (sometimes called NADH)
4H + 2NAD → 2NADH + 2H+
dephosphorylation:
phosphates are transferred from the intermediate substrate molecules to form 4 ATP through substrate-linked phosphorylation
4Pi + 4ADP → 4ATP
pyruvate produced:
the end product of glycolysis which can be used in the next stage of respiration
2 triose phosphate → 2 pyruvate
glycolysis pathway diagram
process of glycolysis