5. Phases and Solution
1/109
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
110 Terms
What determines the phases of matter?
Intermolecular forces (strong = solid, weak = gas)
Solids
Fixed volume and shape; atoms vibrate in place; crystalline or amorphous
Crystalline Solids
Regular arrangement of atoms (lattice); Ex. NaCl
How does H2O solvate NaCl?
Dissociation by separating NaCl via ion-dipole interaction
Lattice Energy
Amount of energy required to separate a solid into component cations and anions (usually very large amount)
Amorphous Solids
No repeating arrangement of atoms (SiO2; silica)
Liquids
Fixed volume, not shape (non-compressible); have viscosity
Viscosity
Resistance of a liquid to flow
Gases
No fixed shape or volume; compressible (no constant density)
How does decreasing volume affect gas density?
Density increases since IMFs increase (particles more close together)
How does increasing volume affect gas density?
Density decreases since IMFs decrease (particles far apart)
Ethane vs. Ethanol
Ethane is more likely to be a gas and ethanol is more likely to be liquid/solid (Ethane only has LDF, Ethanol can H-bond due to OH group)
Affect of high temperature on phases:
High temperatures confer more kinetic energy, making it easier to overcome IMFs → separate
Affect of low temperatures on phases:
Slow down molecules and lower the kinetic energy, making it more likely a molecule will stay together/form IMFs
Affect of high pressure on phases:
Force particles to interact more closely, making it more likely for solids or liquids to form
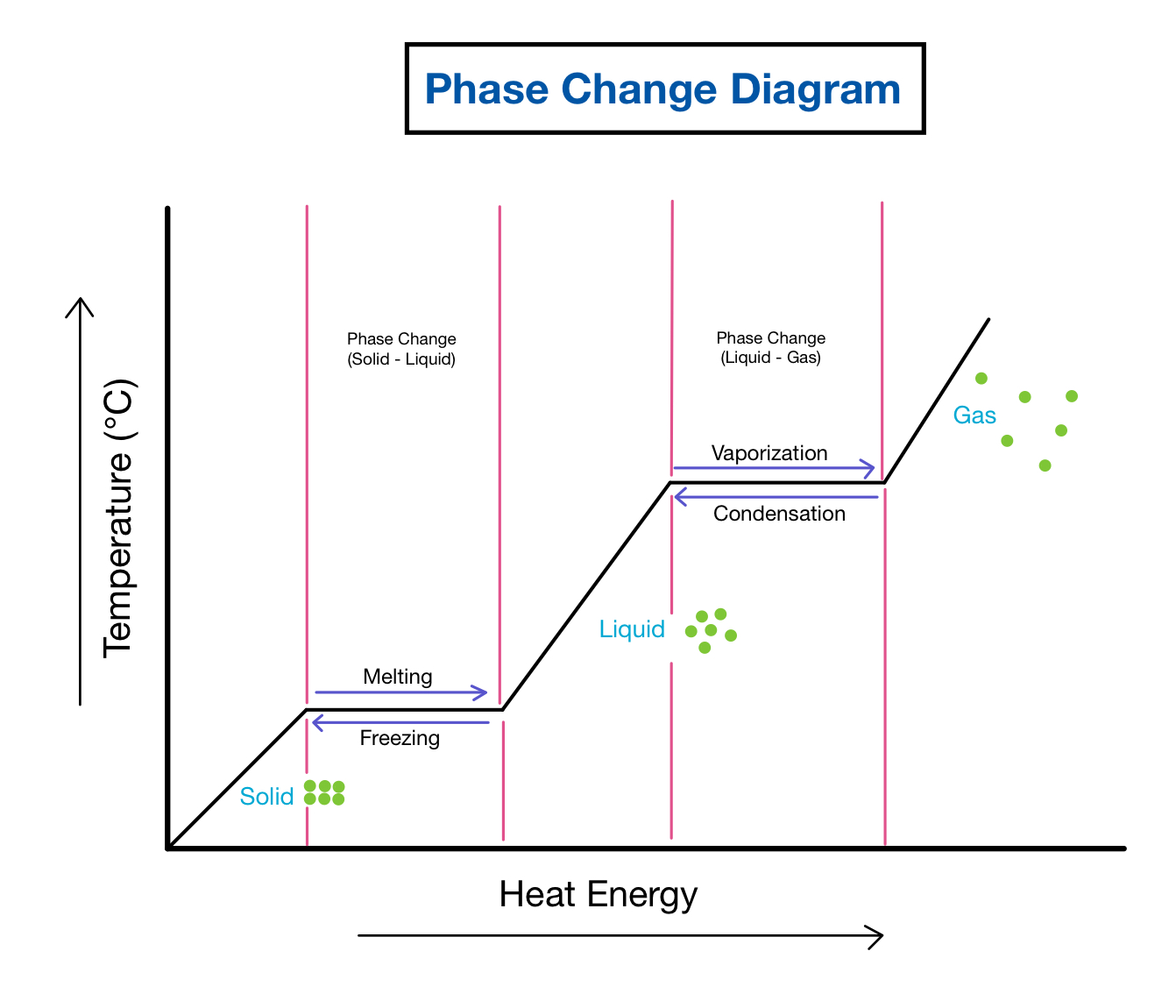
Phase Changes:
Melting/fusion
Freezing
Vaporization
Condensation
Sublimation
Deposition
Melting/fusion
Solid → Liquid
Freezing
Liquid → Solid
Vaporization
Liquid → Gas
Condensation
Gas → Liquid
Sublimation
Solid → Gas
Deposition
Gas → Solid
Which phase changes are endothermic?
Melting/Fusion, evaporation, sublimation (require energy to break apart, +ΔH)
Which phase changes are exothermic?
Freezing, condensation, deposition (release energy upon forming IMF, -ΔH)
Heat of Fusion
Amount of heat used to disrupt intermolecular interactions during melting/fusion at a constant temperature
Melting Point of Water =
0o C (273 K)
Boiling Point of Water
100o C (373 K)
Heat of Vaporization =
Heat required to convert a liquid to gas at a constant temperature
When is temperature constant?
During a phase change (because heat only goes toward breaking IMFs, not increasing temperature)
Units of Heat of Fusion/Evaporation
kJ/mol
What does temperature measure?
The kinetic energy of particles
Specific heat Capacity (c)
Amount of heat required to raise the temperature of 1 unit mass of substance by 1o C (J/g*C)
Specific Heat Capacity of Water
4.184 J/g*C
Why does water have a large heat capacity?
Due to hydrogen bonding; water can take a lot of heat without its temperature changing
qf/qv (heats of fusion/vaporization) =
nΔH; n=moles
q =
mcΔT
When should you use q=mcΔT?
Between phase changes when temperature is changing
When should you use q=nΔH?
When a phase change is occurring.
q = mL
Variation of q=nΔH, where L = latent heat of fusion
Phase Diagrams
Shows the temperature and pressure conditions for phase changes of a substance; solid lines are phase changes
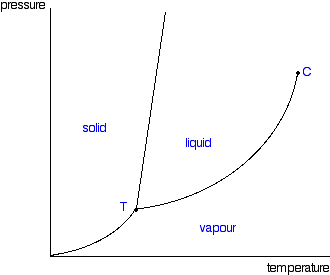
SLUG (Phase Diagram)
Solid → Liquid → Gas in clockwise order
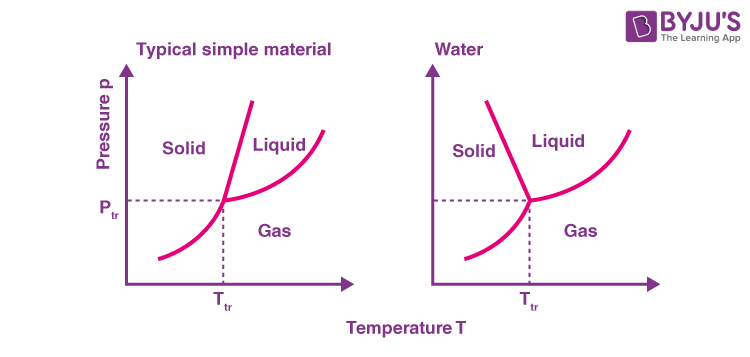
Why is water phase diagram unique?
Ice is less dense than water (unlike other solid forms of substances), so an increase in pressure results in melting rather than freezing
Triple Point in Phase Diagram
Solid, Gas, and Liquid at equilibrium
Critical Point in Phase Diagram
End of liquid-gas interface (liquid is a supercritical fluid with properties of both gas and liquid)
Vapor Pressure
Pressure exerted by gas molecules; at equilibrium, # of molecules escaping to gas phase = # of molecules condensing to liquid
Vapor pressure increases with…
Increasing temperature since KE increases
When vapor pressure = atmospheric pressure,
Boiling point is reached (Ex. water boils at 100 degrees, 1 atm)
STP =
1 atm, 273 K (0 C)
1 atm =
760 mmHg
1 mol gas @ STP =
22.4 L
Ideal Gas Laws (Kinetic Molecular Theory):
Gases are made of atoms/molecules in continuous, random motion
Average KE is directly proportional to temp; all gases have the same KE at a given temp
Gas particles have no volume
Gas particles don’t exert attractive or repulsive forces on each other, only the container
All collisions are elastic, KE is conserved
When do gases deviate from ideal behavior?
High pressures and low temperatures (because likely to become liquid)
When do gases behave ideally?
High temperatures and low pressure
For gas law equations, what temperature scale should you use?
KELVIN always
Celsius → Fahrenheit:
9/5C + 32 (Approximate 2Co +32)
Boyle’s Law (constant temp and moles):
P1V1 = P2V2 (PV = constant)
Charle’s Law (Constant Pressure and moles):
V1/T1 = V2/T2 (V/T = constant)
Gay-Lussac’s Law (Constant Volume and moles):
P1/T1 = P2/T2 (P/T = constant)
What does Boyle’s Law tell us?
If volume increases, pressure decrease; vice versa
What does Charle’s Law tell us?
When temperature increases, volume increases; vice versa
What does Gay-Lussac’s Law tell us?
When pressure increases, temperature increases; vice versa
Ideal Gas Law
PV = nRT (R = .08206 L*atm/K*mol)
Van der Waals Equation (For non-ideal gases):
nRT = (P + an2/V2)(V - nb)
a = attractive force
b = volume occupied by mol of gas
n = moles of gas
Van der Waals Accounts for:
Volume taken up by gas molecules (V - nb)
Attractive forces experienced by gas (P + an2/V2)
Focus on knowing that non-ideal gases can be modeled, and that ideal gases have high T, low P
Mole fraction (Xgas)
Number of moles of a given gas divided by total moles of gas
Xgas =
ngas/ntotal
Derived Mole Fraction (Xgas) =
(ngas/ntotal) = (PgasV/RT)/(PtotalV/RT) = Pgas/Ptotal
Partial Pressure
Pressure that a gas in a mixture exerts if it took up the same volume by itself
Pgas (Partial Pressure) =
XgasPtotal
Dalton’s Law
Total pressure of a mixture equals sum of partial pressures
Ptotal =
ΣPpartial = Pgas1 + Pgas2…
Solution
Homogenous mixture of evenly distributed particles (usually containing solids dissolved in liquids on MCAT)
Dissolution
Solute is dissolved in a solvent (based on solute-solvent interactions - like dissolves like)
Like dissolves like
Hydrophilic (charged or polar) dissolves hydrophilic; vice versa
Molarity (M)
Moles solute/Liters solution (mol/L)
Molality
Moles solute/kg solvent (mol/kg)
ppm (parts per million)
mg/L
ppt (parts per thousand)
g/L
Colligative Properties
Vapor pressure reduction
Boiling point elevation
Freezing point reduction
Osmotic pressure
High vapor pressure =
Low boiling point
Vapor Pressure Reduction
Results in higher boiling points
Raoult’s Law (Vapor Pressure Reduction):
P = XAPAo
P = vapor pressure of solution (reduced)
XA = mole fraction of solvent
PAo = vapor pressure of pure solvent
Boiling Point Elevation:
ΔTb = iKbm
ΔTb = how much boiling point was elevated
i = van ‘t Hoff factor (ionization factor)
Kb = boiling point elevation constant
m = molal solute concentration (mol solute/kg solvent)
Ionization (van ‘t Hoff) Factor
Number of ions a mole of solute
What is i of NaCl?
2; Na+ and Cl-
What is i of C6H12O6 (glucose)?
0; no ions
Osmosis
Net flow of solvent through a semipermeable membrane from high solvent to low solvent (low solute → high solute); trying to lower concentration of solute
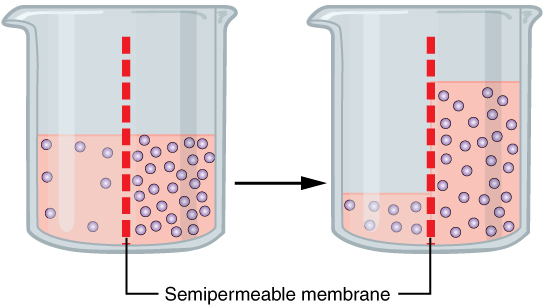
Osmotic Pressure (Π)
Pressure required to prevent osmosis (when solute and solvent are =)
Π (Osmotic Pressure) =
iMRT
i = van ‘t Hoff
M = Molarity (mol solute/L)
R = .08206
T = Kelvin
Solubility
Extent to which various substances will dissolve in a solvent
Solubility equilibrium
Solute dissolves at the same rate is precipitates out of solution
Ksp (Solubility Product Constant) =
[Ion 1]x[Ion 2]x (Solids and liquids not included)
When solution is not in equilibrium, use:
Qsp (same equation, but can compare to Ksp)
High Ksp means…
The compound is soluble
Low Ksp means…
The compound is insoluble
Qsp > Ksp
Supersaturated solution, precipitate will form
Qsp<Ksp
Undersaturated solution, more solute can dissolve
Qsp = Ksp
Solution is at equilibrium (dissolution = precipitation)
Common Ion Effect
Decrease in solubility of a species when a soluble salt is added into the mixture (related to Le Chatlier)
Electrolytes
In solution, charged cations and anions conduct electricity (strong acids, ionic compounds with high Ksp)