Ch. 3 & Ch. 4 Pharmacokinetics and Pharmacodynamics/ Pharmacogenetics
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
71 Terms
Pharmacokinetics
drug movement through body what body does to drug
Pharmacodynamics
what drug does to the body
drug name categories
1.) chemical
2.) generic
3.) trade
chemical name
based upon the compounds that make up a drug. Because this name uses chemical nomenclature, it can look and sound complicated, making it inappropriate for everyday use
generic name
elected by the United States Adopted Names Council. The generic name is unique for each drug and is based upon the pharmacologic and/or chemical classification of the drug. Often the generic names for drugs within the same pharmacologic class have the same last syllables.
trade (brand) name
chosen by the manufacturer of the drug. The trade name is selected for marketing and is therefore simpler and easier to remember and pronounce than the generic name. Because the trade name is owned by the manufacturer, it is also known as the proprietary name. Trade names always begin with an upper-case letter. The same drug made by different manufacturers will have different trade names but only one generic name.
bioequivalent
the generic drug has the same pharmacokinetic and pharmacodynamic properties as the brand name drug; they share the same therapeutic effects and adverse drug event profile.
equivalent
a less than 20% variance in pharmacokinetics from the brand name drug
drug standards
Dosage
Drug forms
Drug substances and excipients
Biologics
Compounded preparations
Dietary supplements
United States Pharmacopeia and the National Formulary (USP-NF)
sets drug standards for the United States
The Federal Food, Drug, and Cosmetic Act of 1938
empowered the FDA to ensure a drug was safe before marketing. It is the FDA's responsibility to ensure that all drugs are tested for harmful effects; it also required that drugs be labeled with accurate information and have detailed literature in the drug packaging that explains adverse effects.
The Food and Drug Act of 1906
set the standards for drug quality and purity in addition to strength and gave birth to the FDA
Kefauver-Harris Drug Amendment to the 1938 Act (1962)
tightened controls on drug safety, especially experimental drugs and required that adverse reactions and contraindications be labeled and included in the literature
The Controlled Substances Act of 1970
designed to remedy the escalating problem of drug abuse, included several provisions :
- Promotion of drug education and research into the prevention and treatment of drug dependence
- Strengthening of enforcement authority
Establishment of treatment and rehabilitation facilities
- Designation of schedules, or categories, for controlled substances according to abuse liability
Dietary Supplement Health and Education Act of 1994
established labeling requirements for dietary supplements and authorized the FDA to promote safe manufacturing practices. It classified dietary supplements as food
Schedule of Categories of Controlled Substances
-The abuse potential is greatest with schedule I drugs and lowest with schedule V drugs.
-Schedule I drugs are not approved for medical use; they include street drugs and have a high potential for physical and psychological dependence.
-Schedule II through V drugs have accepted medical use.
-Drugs may be listed in more than one schedule category when combined with another drug.
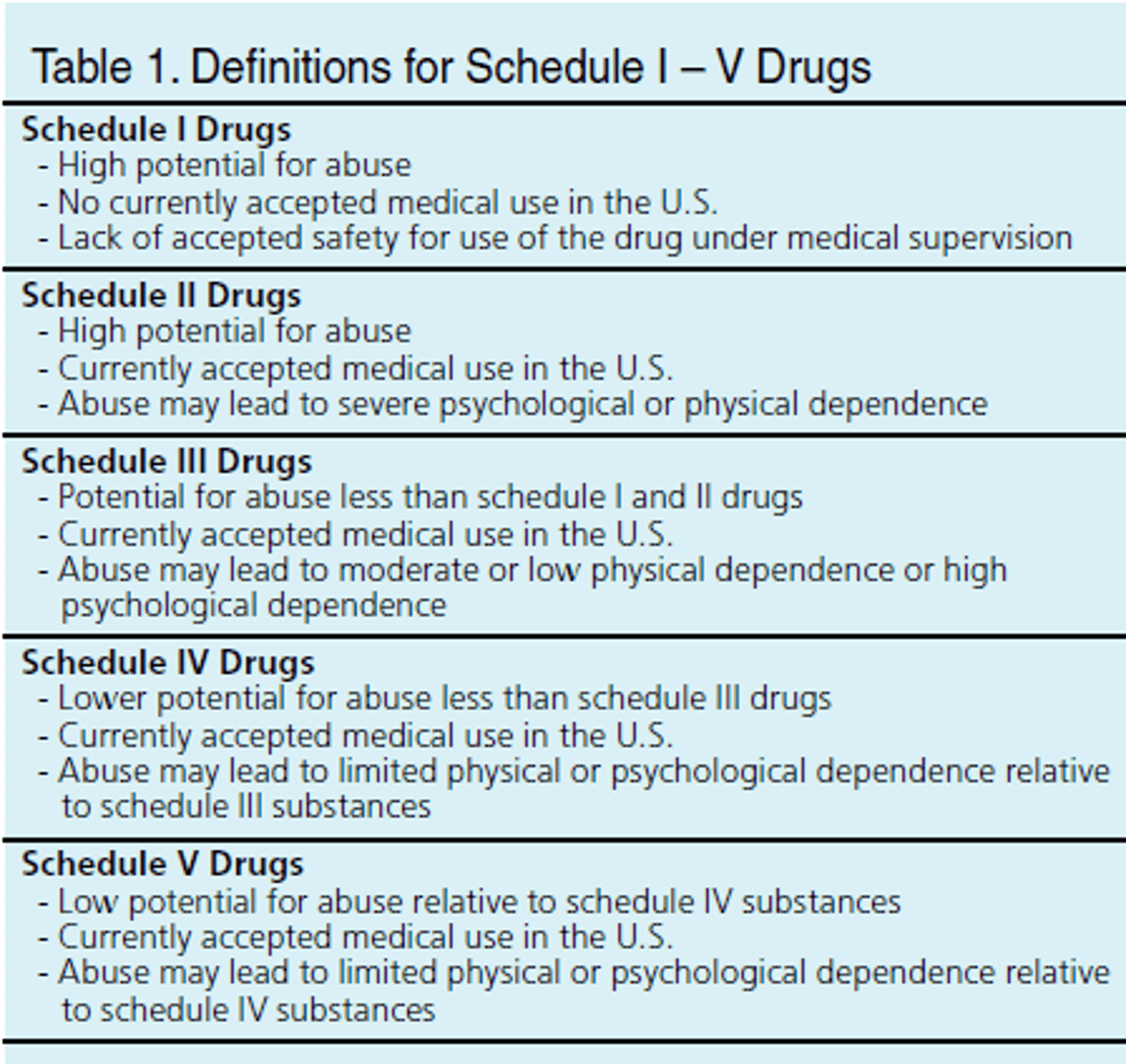
Which schedule of controlled substances has the greatest abuse potential?
Schedule I drugs
Which schedule of controlled substances has the lowest abuse potential?
Schedule V drugs
Are Schedule I drugs approved for medical use?
No, they are not approved for medical use.
What types of drugs are included in Schedule I?
Street drugs with a high potential for physical and psychological dependence.
Do Schedule II through V drugs have accepted medical use?
Yes, they have accepted medical use.
Can drugs be listed in more than one schedule category?
Yes, when combined with another drug.
What are Schedule I substances defined as?
Drugs, substances, or chemicals with no currently accepted medical use and a high potential for abuse.
Give examples of Schedule I substances.
Heroin, LSD, marijuana, MDMA, methaqualone, and peyote.
What are Schedule II substances defined as?
Drugs, substances, or chemicals with a high potential for abuse, with use potentially leading to severe psychological or physical dependence.
Give examples of Schedule II substances.
Combination products with less than 15 mg of hydrocodone per dosage unit, cocaine, methamphetamine, methadone, hydromorphone, meperidine, oxycodone, fentanyl, dextroamphetamine, dextroamphetamine/amphetamine, and methylphenidate.
What are Schedule III substances defined as?
Drugs, substances, or chemicals with a moderate to low potential for physical and psychological dependence.
Give examples of Schedule III substances.
Products containing less than 90 mg of codeine per dosage unit, ketamine, anabolic steroids, and testosterone.
What are Schedule IV substances defined as?
Drugs, substances, or chemicals with a low potential for abuse and low risk for dependence.
Give examples of Schedule IV substances.
Alprazolam, carisoprodol, diazepam, lorazepam, zolpidem, and tramadol.
What are Schedule V substances defined as?
Drugs, substances, or chemicals with lower potential for abuse than schedule IV and consist of preparations containing limited quantities of certain narcotics.
Give examples of Schedule V substances.
Cough preparations with less than 200 mg of codeine per 100 mL, codeine/guaifenesin, diphenoxylate/atropine, difenoxin/atropine, and pregabalin.
What did the FDA replace in 2015 regarding pregnancy categories?
The FDA replaced the lettered pregnancy categories A, B, C, D, and X on prescription drug labeling.
What are the new subsections introduced by the FDA for pregnancy labeling?
The new subsections are Pregnancy (includes Labor and Delivery), Lactation (includes Nursing Mothers), and Females and Males of Reproductive Potential.
When will prescription drugs and biologic products submitted after June 30, 2015, use the new labeling format?
They will use the new format immediately.
How will labeling for prescription drugs approved on or after June 30, 2001, transition to the new format?
Labeling will be phased in gradually.
Will pregnancy labeling for OTC drugs change after the 2015 FDA update?
No, pregnancy labeling for OTC drugs will not change.
What is Section 8.1 of the new FDA pregnancy labeling?
Section 8.1 covers Pregnancy and includes Labor and Delivery.
What does Section 8.2 of the new FDA pregnancy labeling cover?
Section 8.2 covers Lactation and includes Nursing Mothers.
What new section was added for Females and Males of Reproductive Potential?
The new section includes Pregnancy testing, Contraception, and Infertility.
What is a requirement for drug labels as new information becomes available?
There is a requirement to update the label as new information becomes available.
Black Box Warning
A notice that a drug may produce serious or even life-threatening effects in some people in addition to its beneficial effects.
This is the strongest safety warning a drug can carry and remain on the market.
Drug absorption
drug movement, disintegration, dissolution
Factors affecting absorption
acidity, motility, food in stomach, stress, temperature, hormonal changes
Constipation
slows down motility
Foods cause absorption
by awakening
Stress effects the gut
slows down, less blood profusion
Onset
Time it takes for drug to reach the minimum effective concentration
Peak
highest concentration in blood
Duration
Length of time drug exerts a therapeutic effect
Therapeutic Drug Monitoring
monitoring drug levels to ensure efficacy and safety (peak & troughs)
Peak drug level
highest plasma concentration of drug at a specific time
Trough drug level
lowest plasma concentration of drug
Agonist
activate receptors, producing a desired response
Antagonists
prevent receptor activation, block response
Side effects
secondary drug effects
Adverse reactions
mild to severe, unintentional, unexpected, undesirable effects
Drug toxicity
drug level exceeds therapeutic range
Tolerance
decreased responsiveness to drug over course of therapy, requires high dose to achieve same therapeutic response
Tachyphlaxis
acute, rapid decrease in response to a drug
Placebo Effect
drug response not attributed to drugs chemical properties
Additive drug effects
sum of effects of two drugs
Synergistic drug effects and potentiation
effects of two drugs in much greater than effects of either drug alone
Antagonistic drug effects
one drug reduces or blocks effect of the other drug
Drug-nutrient interactions
Food may increase, decrease, or delay drug response.
Drug-laboratory interactions
Drugs may cause misinterpretation of test results.
Drug-induced photosensitivity
Drug induced skin reaction caused by sunlight exposure
Pharmacogenetics
Study of how a patient's genomes affect drug response
- Helps individualizeoptimal drug treatment regimens
- Helps decrease drug reactions
- Promotes drug regimen adherence
- Reduces overall healthcare costs
Patients who benefit most from pharmacogenetics
Those taking multiple prescription drugs
Those not responding to the current therapy
Those having adverse drug reactions
Those taking black box warning drugs
Notes about Pharmacogenetic Testing
Pharmacogenetic testing is not available for all drugs
One single test doesn't determine how a patient will respond to all drugs
International Difference
- language barrier may prevent obtaining the same drug
- trade names for the same drug can differ between countries
- drugs with the same trade name may have different active ingredients in different countries