Reactions of Alkenes
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
What are the two possible products in the reaction of propene with HBr?
The bromine atom can be added to either the first carbon or the second carbon
You can therefore produce either 1-bromopropane or 2-bromopropane
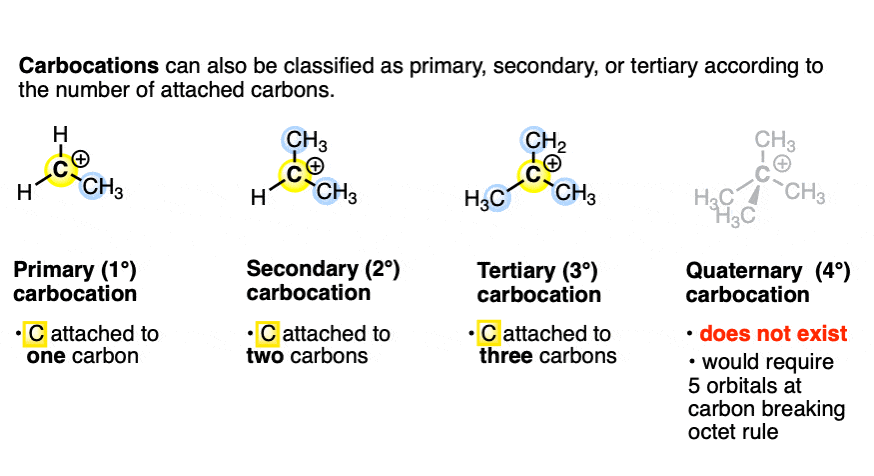
What are the three possible carbocations?
Primary carbocation- bonded to 1 alkyl group
Secondary carbocation- bonded to 2 alkyl groups
Tertiary carbocation- bonded to 3 alkyl groups
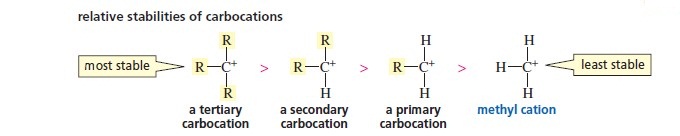
What is the trend in stability of the carbocations?
Carbocations with more alkyl groups are more stable because the alkyl groups feed electrons towards the positive charge which helps stabilise the positive charge
More stable carbocations are much more likely to form
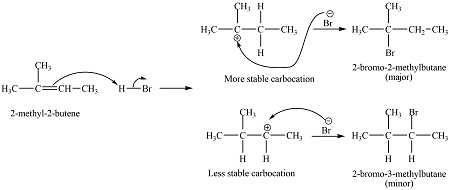
How does HBr react with 2-methylbut-2-ene?
The secondary carbocation is less stable as it only has 2 alkyl groups. It forms less often
The tertiary carbocation is more stable as it has 3 alkyl groups. It forms more often
2-bromo-3-methylbutane is the minor product
2-bromo-2-methylbutane is the major product