Physical chemistry Atomic structure (3.1.1)
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
Proton
relative mass = 1 relative charge= +1 location = nucleus
Electron
relative mass = 1/1840 rel charge = -1 shells surronding nucleus at fixed energy levels
Neutrons
relative mass = 1 relative charge = 0 location = nucleus.
Proton number
Number of protons in the nucleus of an atom
Mass number
The total protons and neutrons in any given isotope
Calculating the number of neutrons
mass number = proton number + neutron number
The atomic model
In 1803 John dolton described atoms as solid spheres and that different spheres made up different elements. In 1897 J.J Thompson concluded from his experiemnts that atoms must contain smaller negatively charged particles called electrons. Creating the plum pudding model
Rutherford's model
Students of Rutherford carried out experiements in which thin metal foils were bombarded with alpha particles (positively charged helium nuclei). The crucial experiment involved firing alpha particles at a thin metal foil. Some particles appeared to rebound back from the foil to ths source, most passed straight through and some partially deflected.
'Rutherford's further studies (radius of atom, charge of protons, space of atom)
The studies showed that the radius of the atom is about 10 thousand times greater than the radius of the nucleus.The proton exist in the nucleus of all atoms. Its charge is equal in size but opposite to that of an electron. An atom is mostly space.
Bohr's Model
Bohr proposed a new model with the following prinicples. Electrons only exist in fixed orbits and not anywhere in between. Each shell has a fixed energy. When an electron moves between shells electromagnetic radiation is emitted or absored. Since the energy of the shells is fixed the radiation will have a fixed frequency
Other atomic models
Scientists later discovered that all the electrons in the same shell had the same energy so it was refined to include sub shells. The most accurate model today is based on Quantum Mechanics
Isotopes
Atoms of the same element that have different numbers of neutrons
Why is mass spectrometry used?
To determine the relative abundance of the isotopes of elements. Once the relative abundance is know it is sample matter to calculate the Ar of an element. Mass spectrometry can be used to identify both elements and RAM
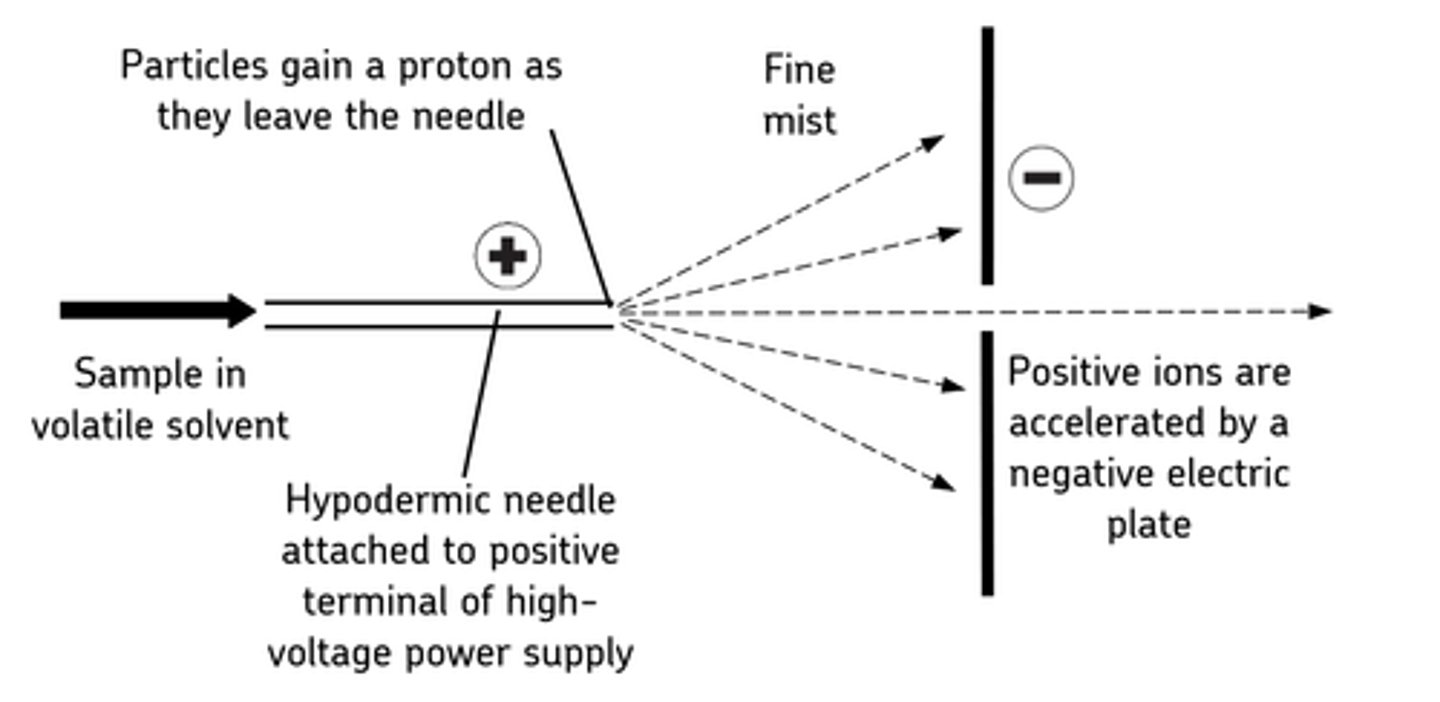
Electrospray ionisation (1st stage of mass spectrometruy)
The sample of the element is first dissolved in a polar solvent and pushed through a small nozzle at high pressure. Positive ions are produced by applying a high voltage to it, causing the dissolved particles to be ionised by gaining a proton from the sovent as they leave the needle. The ionised particles are then separated from the solvent when it evaporates leaving a gas made up of positive ions.
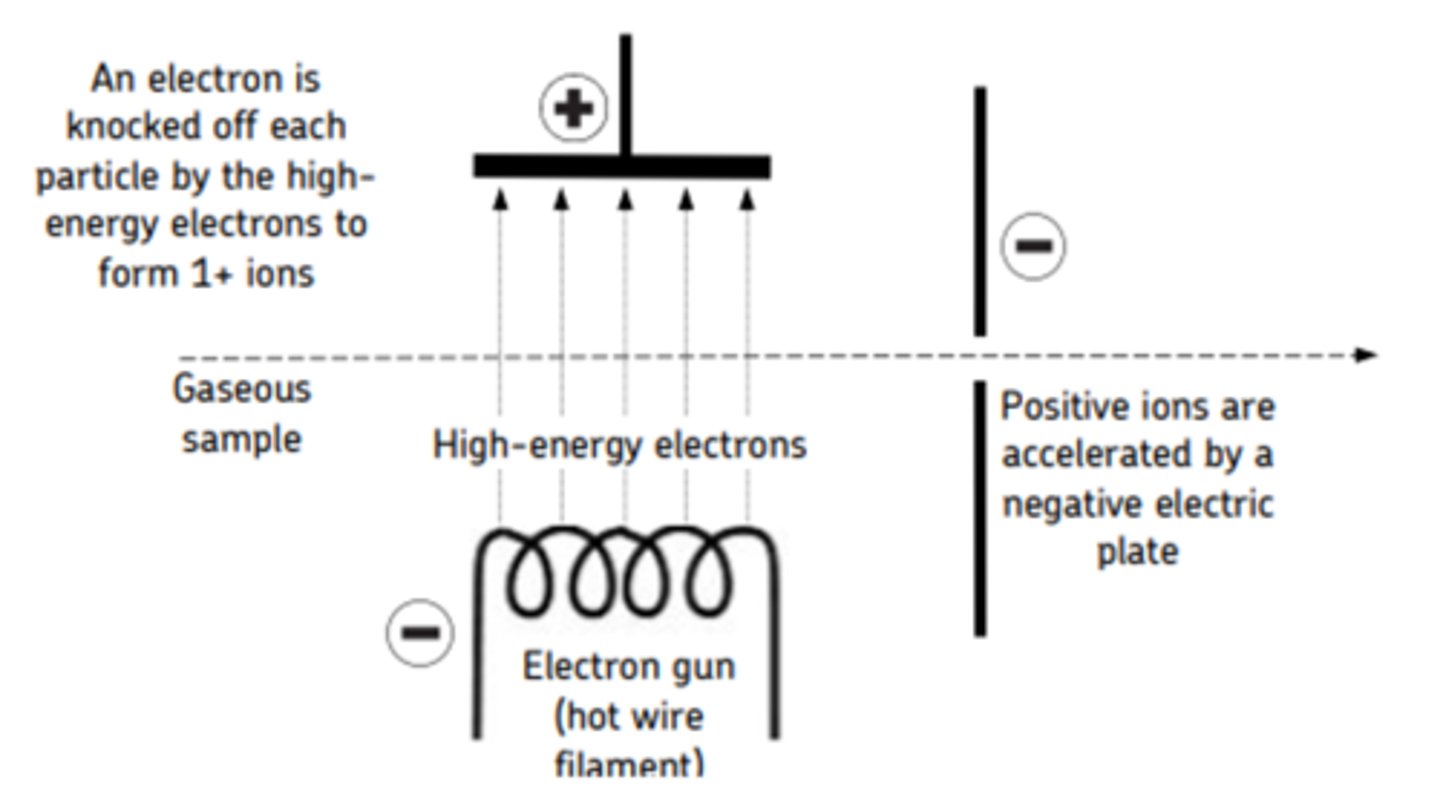
Electron impact ionisation (1st stage of mass spectrometry)
The sample being ionised is vaporised and then high energy electrons are fired at it from an electron gun which is a hot wire filament with a current through it that emits electrons. This usually knocks off one electron from each particle producing a 1+ ion
When electron impact ionisation used?
Used for elements and substance with low relative formula masses. Molecules ionised this way are molecular ions
Why is a doubly charged ion sometimes produced during electron impact ionisation
This involves dislodging 2 outer electrons but requires a very large amount of energy and is unlikely. Keep energy of electrons to the minimum required to form 1+ ions.
Acceleration ( 2nd stage of mass spectrometry)
The positive ions are then accelerated by passing them through an electric field ( 2 negatively charged plates. The produces a beam of particles with very similar Kinetic Energy. The ions with a lower mass to charge to ration experience a greater accelerration because they are given as much energy as ions with a greater m/z but they are lighter so accelerate more.
Kinetic energy equation
KE=1/2mv^2
Flight tube (3rd stage of mass spectrometry)
The ions leave the electric field with a constant speed and constant kinetic energy. They entr a region with no electric field and drift through it at the same speed as they left the electric field. So ions with lower m/z ratios will be drifting at higher speeds.
Detection ( 4th stage of mass spectrometry)
Since ions with a lower ratio travel through the drift region at higher speeds they reach the detector in less time than ions with higher ratios. When the ions hit the detector ( a charged plate) electrons flow from the negative plate to the positive and a current is induced. The detector will record how long it took the ions to pass through spectrometer and the calculate the m/z ratio of that particular ion. More ions hitting detector = More current = more abundance.
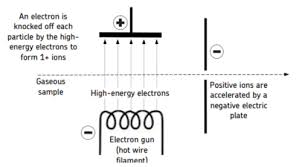
Electron impact (with image)
High energy electron dislodges from a gaseous atom
Ionic equation for ionisation in mass spectrometry with potassium (1st ionisation and 2nd)
K(g) → K+(g) + e-
K+(g) → K2+(g) + e-
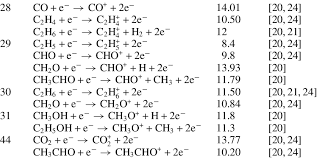
Examples of some more ionic equations in electron impact
Why is a doubly charged ion sometimes produced in electron impact
If enough energy is used 2 out electrons can be dislodged instead of just one. But this is unlikely as in chemical reactions chemists want to be sustainable and not use as much energy
What is fragmentation in terms of electrons
This is where a high speed electron hits an outer electron splitting the electron creating a doubly charged ion
Kinetic Energy equation
1/2mv²
Rearranging Kinetic energy equation to get velocity
root 2KE/m
What does the velocity of ions in a mass spectrometer depend on
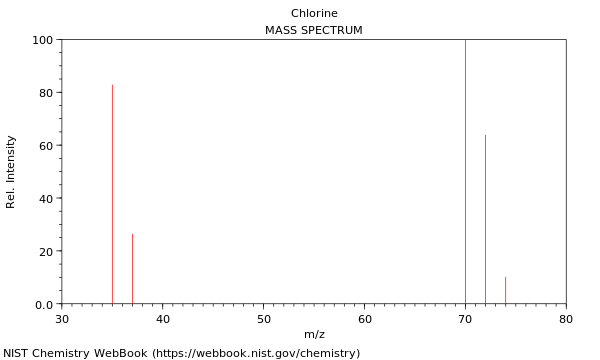
All the particles have the same K.E after leaving the negative plates the velocity depends on the mass (m/z ratio) e.g 35Cl+ has a greater velocity than 37Cl+
What happens in the flight tube
Ions will deflect due to electromagnets and there is no electron field so the ions drift
What is the relationship between time of flight and mass of the ions
the time of flight is proportional to the square root of the mass of the ions
Chlorine mass spectrun
First ionisation energies first part of diagram
How to calculate RAM (Relative atomic mass)
Abundance of isotope 1 x mass of isotope 1 (or m/z ratio) + abundance of isotope 2 x mass of isotope 2 (or m/ratio)/ total abundance
Describe how ions are formed ina time of flight mass spectrometer
Sample dissolved in a polar volatile solvent forced a syringe at hig prssures, the positive terminal of high voltage supply attatched sample gains H+ from solvent
Why is it neccesary to ionise molecules when measuring their mass in a TOF mass spectromete
For detection (e- transfer from -ve plate plate to +ve ion induces a current)
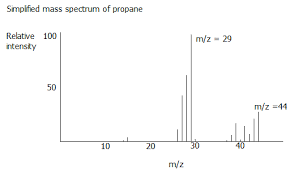
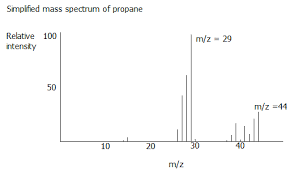
Diagram of propane in mass spectrum
The molecular ion peak tells you the Mr of the compound
ELectrospray ionisatioon Mr determination explained (with diagram)
If a molcules such as a protein is ionised by electrospray ionsation using protonation the m/z peak is for MH+ so the relative molecular mass is one less than the m/z value e.g if the peak was 531 then the mr would be 530
Why is electron arrangement important?
The way in which electrons are arranged determines the chemical reactivity of an atom. E.g the general trend of reactivity in group 1
What are orbitals
Electrons move around the nucleus in very definite energy levels (shells). Saying that electrons move around the nucleus in not enough. You cannot predict the position of an electron in an atom but you can say where that electron is most likely to be found. These regions are known as orbitals
Orbital types
s,p,d,f
S orbital explained
An electron in an s orbital can be considered to be a diffused cloud of negative charge distributed throughout the volume of a sphere.
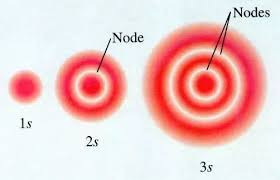
Graph displaying electron density and distance from nucleus

P-orbital image
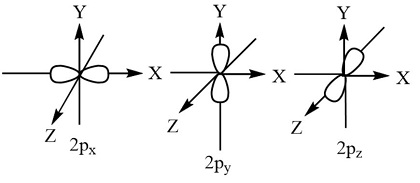
Hund’s rule
electrons prefer to occupy orbitals on their own and only pair up when no empty orbitals of the same energy are available
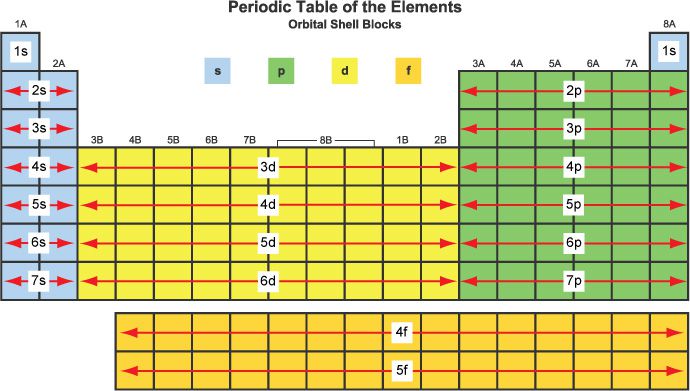
S P D F blocks on periodic table
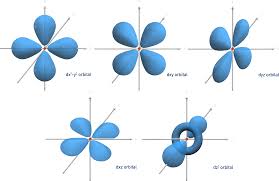
d orbital image
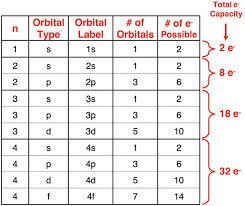
Energy levels, number of orbitals subshells and type of subshell (number = energy level/shell
Why is He atom smaller than H?
due to protons in the nucekus and electrons in the same energy level and subshell
Sequence of orbitals
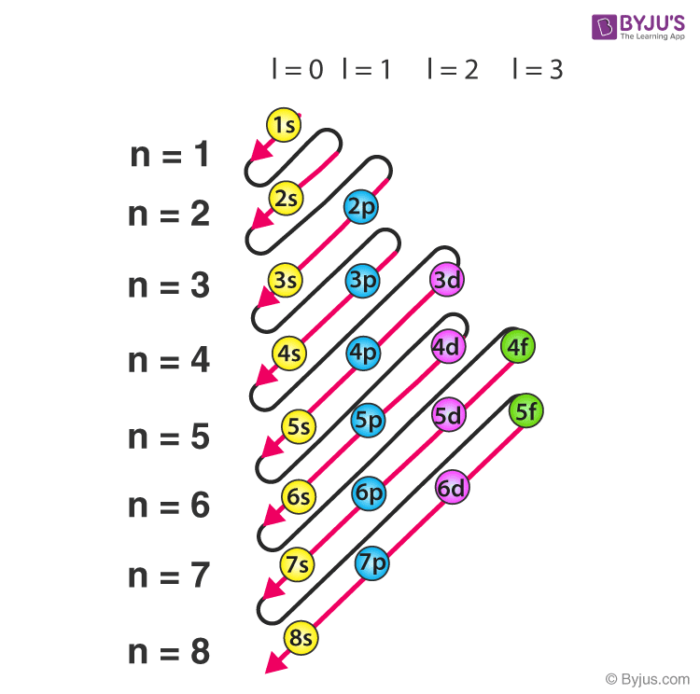
Exceptions to these sequences of orbitals
Cr: 1s2 2s2 2p6 3s2 3p6 4s1 3d5. Instead of: 4s2 and 3d4 at the end.
Cu: 1s2 2s2 2p6 3s2 3p6 4s1 3d10. Instead of 4s2 and 3d9 at the end.
Whys is the electron have a greater probability of being found further from the nucleus with 1s and 2s and 3s orbitals
The 2s orbital like the 1s orbital is spherically symmetrical around the nucleus. A 2s orbital differs from the 1s orbital that it is larger. 3s orbitals being larger than 2s orbitals
All difference 2nd energy level orbitals (p orbitals0
2px 2py 2pz (these all hold 2 electrons) so the 2nd p orbital holds 6 electrons
Blocks and how many electrons they hold
s = 2e- p=6e- d=10e-
How do you calculate the electron configuration?
the number of electrons the element has and then count out of the orbitals. E.g N = 7 so 1s2, 2s2, 2px1,2py1,2pz1, 2 + 2 + 1 + 1 + 1 = 7 the 2 p orbitals only have 1 electron due to hunds rule (fill unoccupied orbital first)
what do the arrows represent in the orbital sequence diagram
electron spin (if 1 electron is placed along each orbital in a subshell then there will be repulsion)
State 2 differences between the plum pudding model and the model used today
Plum pudding had positive charge evenly distributed whilst modern model had positive charge mostly in the centre of the atom (nucleus). In plum pudding the electrons were randomly scattered in a positive sea whilst in the modern model the electrons orbit the nucleus at fixed distances
Image of first ionisation energies in group 2
Be = 900 Mg= 740 Ca = 590 Sr= 548 Ba= 502
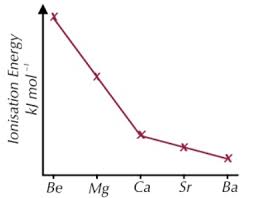
What is the trend of first ionisation energies in group 2 and why is it caused?
Decreases down the group due to bigger atomic radius more shileding and weaker attraction between nucleus and electron
Why is 4s filled before 3d
This is becuased if you fill in 3d first the electrons will be in similar spins so they will repel each other which reduces stability
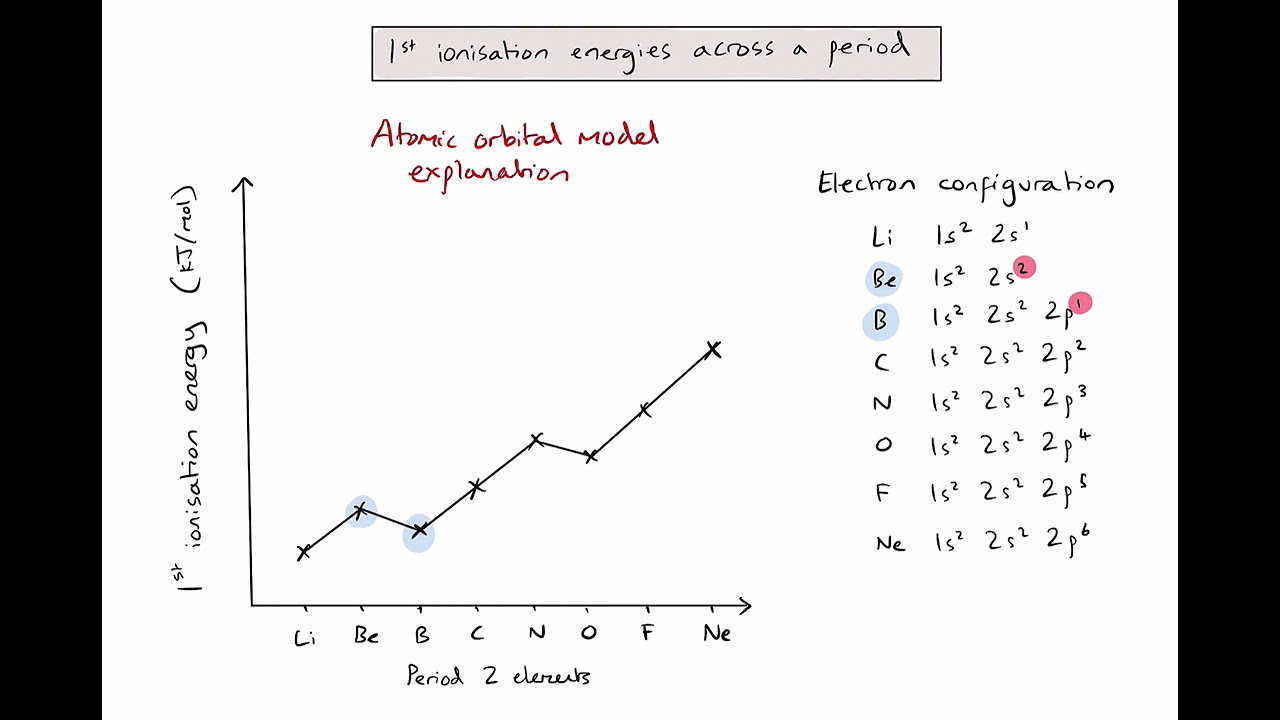
Ne to Na ionisation energies explained
Large decrease in 1st I.E from Ne - Na , in Na outer e- is lost from an energy level further from the nucleus so there is more shielding and so less attraction from the nucleus to the outer electron therefore less energy required to remove it. The reason why the ionisation energy dips is due to the different subshells between Ne and Na. Ne outer e - on 2p6 orbital whilst Na its 3s1
Na to Mg ionisation energies explained
Small increase in 1st I.e from Na to Mg. Mg has one more proton in the nucleus Outer electron in sam e energy level and subshell so same / similar shielding. So greater attraction from the nucleus to the outer electron so more energy required to remove it.
Mg to Al ionisation energies explained
Small decrease in 1st I.E from Mg to Al. In Al outer electron lost from subshell further from nucleus (or of higher energy). 3p instead of 3s so less attraction from nucleus to outer electron and less energy required to remove it. (Mg from S -orbital whilst Al from p - orbital)
Al to Si to P ionisation energies explained
Increase in 1st I.E from Al to Si to P due to outer electron in same energy level and subshell so similar shielding an increase in number greater attraction from nucleus to protons from Al to Si to P
P to S ionisation energies explained
small decrease in 1st I.E from P to S due to pairing in p-subshell for first time. Causing repulsion between electrons exception to trend (any gp5 -gp6)
S to Cl to Ar
increase in 1st I.E from S to Cl to Ar. Due to an increase in number of protons from S to Cl to Ar. Outer electron in same energy and subshell so similar shielding greater attraction from nucleus to outer electron so more energy required to remove it. This is also the general trend across a period
How does a period change attributes of an atom
As you go across in a period (left to right). The atomic radius decreases more protons and stronger attraction between nucleus and electron. Resulting in similar shielding. The outer electron stays in the same shell
Why is there repulsion between group 5 and 6
There is paired electrons in p - orbitals paired repel.
How are elctron lost during ionisation energies
They are lost stepwise. I.E =x+(g) → x² + e-
I.e x2+(g) → x3+ (g) + e-
What does the biggest jump in a trend of ionisation energies mean?
the
Equation that links avagadros constant, RFM and mass
RFM / advogadros constant = mass in kg
Why does selenium deviate from the general trend in first ionisation energies for period 4 elements
First time pairing of electrons in the 4p orbital results in paired electrons repelling each other so selenium will increase in first ionisation energy deviating from this trend as it requires more energy in order to fight the force of repulsion in the orbital.
Why is the first ionisation energy of sulfur less than the first ionisation energy of phosphorus
Sulfur has 4 electrons in its 3p subshell whilst phosphorus has 3, this means sulfur has pairing in the 3px subshell (hunds rule) which causes more repulsion and as a result it is easier to lose the electron
Why is 2nd ionisation energy usually higher than 1st ionisation energy
electron is being removed from positive ion therefroe this ion is closer to the nucleus and requires more energy to remove.