Model quantum mechanical problems:atomic systems
0.0(0)
Card Sorting
1/20
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
1
New cards
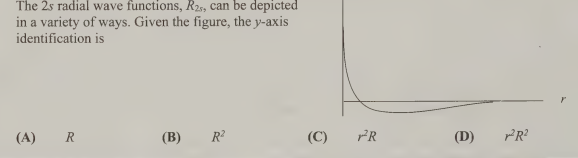
A- R because the radical function for 2s orbital has one node
2
New cards

B-2
3
New cards

C-4.11 × 10^15 Hz
4
New cards
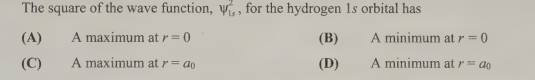
A- the maximum at r=0 (It would be B for r²wavelength² 1s)
5
New cards

A- the energy only depends on the principal quantum number(n) and not on the angular momentum (l) or quantum number(m1)
6
New cards

A-the energy of a state does not depend on the azimuthal quantum number only the principal quantum number(n)
7
New cards
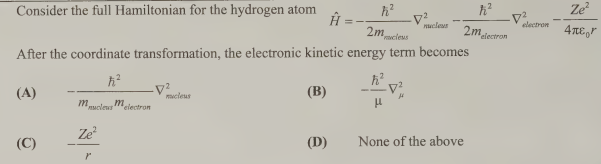
B
8
New cards

D
9
New cards

D because the triplet is lower
10
New cards

B- spin-orbit coupling
11
New cards

D
12
New cards
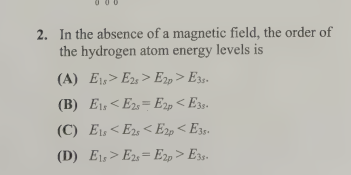
B- E1s<E2s=E2p<E3s
13
New cards

B- Ep+1<Ep0<Ep-1
14
New cards

D- ± 1h
15
New cards

C- r²R² against r
16
New cards

C- pi
17
New cards

A- 3s to 3p
18
New cards

C- 6s6p5d^10 ³p1 to 6s²5d^10 1S0
19
New cards

B- the electron-electron repulsion term
20
New cards

B- 2 angular nodes and 1 radial node
21
New cards

D-taking linear combinations of the imaginary functions