NMSK - week 7 cranial nerves and neurological clinical examination
1/143
Earn XP
Description and Tags
LOs for this week: Explain the concept of lateral inhibition Describe the functional arrangement of retinal ganglion cells Describe the higher order processing of visual information Discuss possible causes of seizures and know what epilepsy (epilepsies) is (are) Explain the general mechanisms of action of anticonvulsant drugs Specify the anticonvulsant drugs mainly used in the treatment of epilepsy in small animDescribe a seizure and explain what is happening in the brain during a seizure Give examples of other conditions that an owner could confuse with a seizure List the intra- and extra- cranial causes of seizures Use the results of the neurological examination to localise the lesion Explain the mechanism of action of two commonly used anti-epileptic drugs Explain the concept of half-life and the clinical implication for dosing regimes. List the general pathological processes represented by the VITAMIN D mnemonic Understand the importance of a problem list in addressing clinical scenarios Understand the concept of the sign time graph. Describe the course of the cranial nerves as they pass from the brain into the extracranial space
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
144 Terms
what is meant by the association cortex?
a series of interconnected regions that are responsible for ‘higher functions’ including controlling voluntary initiation of function and movements
what areas can we map on the brain?
visual
auditory
somatosensroy
olfactory
motorDescribe the course of the cranial nerves as they pass from the brain into the extracranial space
what are the 3 building blocks for visual processing?
photoreceptors (in the eye that contain rods and cones)
visual pathways (connects eye to brain)
Brodman’s area 17-19, visual cortex (in the occipital lobe)
how can we use experimental electrophysiology
record from and label cells using microelectrons (look at what stimuli result in what outcome)
functional MRI - look at the changes between oxygenated and deoxygenated blood
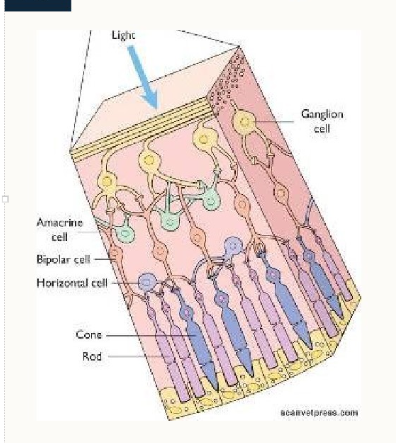
what is the structure of the retina?
3 layers:
Ganglion cells - produce white matter tract to transmit information to the brain via the optic nerve
Bipolar cells, amacrine cells (connect widely) and horizontal cells (connect horizontally) - opportunity for processing at the level of the retina before transmission to the cortex
Photoreceptors - transduction of photon energy into membrane depolarisation and action potentials (rods and cones)
what is retinal electrophysiological recording?
one to one relationship at the receptor level
complex multimodal relationship at the ganglion cell layer
there’s not a direct line from photoreceptor cells to optic cells
what are the modes of ganglion cell responses?
single cell recording with small visual stimulus
small field (fovea)
large field (periphery)
lateral inhibtion
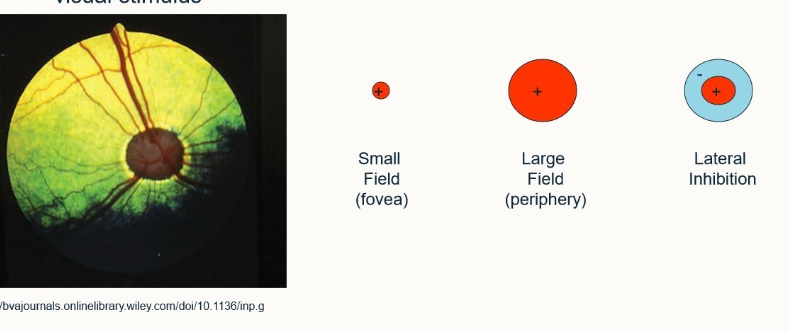
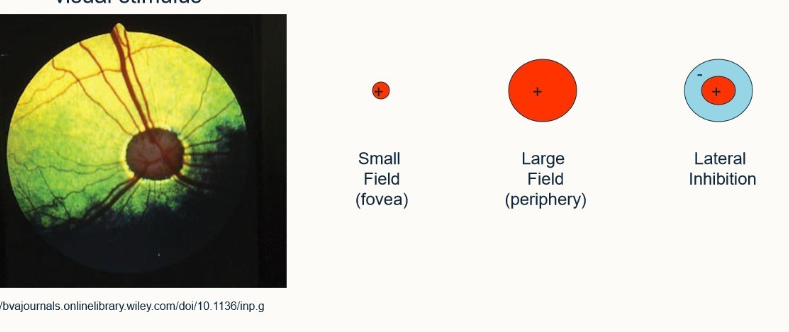
what is meant by lateral inhibition?
The ganglion cell is stimulated by a central region but around this region there’s an area of inhibition, which if stimulated inhibits anything else within the field of view
improves edge detection
improves localisation
if you shine a light on it/leave it in darkness, shows a normal response. If they’re on the edge of the light source they show an enhanced response and if they’re in the corner, show an even more enhanced response - enables us to observe edges.
what is meant by the gain and transcience of ganglion cell response?
gain = horizontal cells, wide receptive fields (field adaption)
Transcience - altered temporal responses
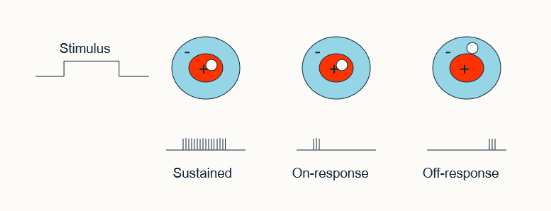
what is the proportion of sustained vs transient ganglion cell response
80:20%
how many different modes of ganglion cell responses reported in the cat are there?
23 including response to moving stimuli
what is the lateral geniculate nucleus
similar receptive fields as ganglion cells
just a relay bod
different modalities and sides kept separate and in ordered patterns
gating from other regions of the brain can be demonstrated
In this nucleus the neurones synapse with the optic radiation nerve → occipital cortex. Very little processing takes place here, however there is the opportunity for the brain to produce some gating to this information
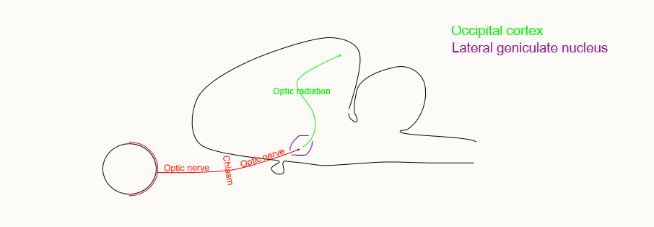
where do the optic radiations go?
into Brodman’s area 17
pyramidal cells which integrate information from the geniculate radiation project to surrounding areas
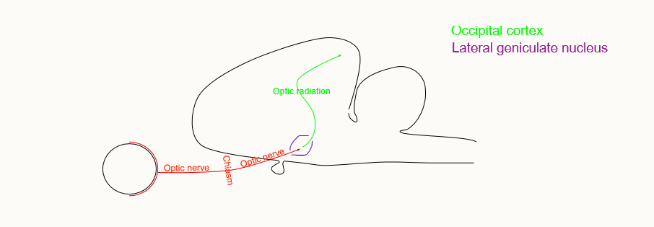
what is Brodman’s area 17
novel forms of visual fields:
simple cells - can be mapped with spots of light
complex cells - respond to bars of light/an edge of specific orientation
hypercomplex cells (end stopped complex) - bar of light must be correct length
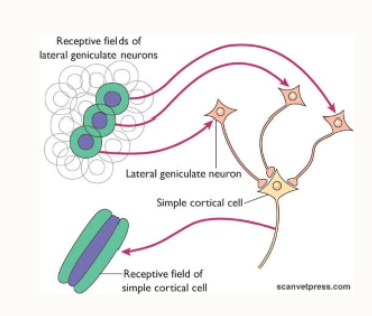
where does the information go from Brodman’s area 17?
into areas 18,19 and beyond (association cortex)
localisation vs recognition
increasingly difficult to identify recognition patterns
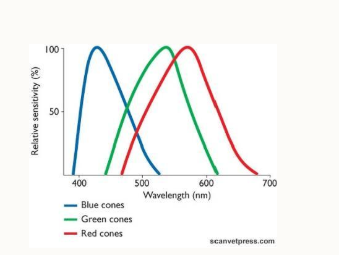
How do we see colour?
this is an additional tier of information processed by the brain
3 colour cones: red, green and blue
define a seizure
temporary, abnormal, electro-physiological phenomena of the brain, resulting in abnormal synchronisation of electrical neuronal activity. Due to temporary abnormal electrical activity of a group of brain cells
define epilepsy
a disease of the brain characterised by an enduring predisposition to generate epileptic seizures (it’s a description, not a diagnosis)
applied as having 2 unprovoked epileptic seizures greater than 24 hours apart
what are convulsions
sudden and often violent motor activity of cerebral or brainstem origin
may also occur in the absence of an electrical cerebral discharge e.g. in response to hypotension, hypoxia
not all epileptic seizures cause convulsions
how is epilepsy characterised?
recurrent episodes of paroxysmal brain dysfunction due to a sudden, disorderly and excessive neuronal discharge
what % epileptic dogs are there?
0.6-0.75
what are the 2 kinds of epilepsy?
focal and generalised
what are 3 causes of epilepsy?
idiopathic
structural
unknown
when does epilepsy become a full seizure?
when it moves from being localised to affecting a large area of the brain
what’s an example of a structural cause of a seizure?
intracranial/cerebral pathology:
vascular
inflammatory/infectious
traumatic
anomalous/developmental
neoplastic and degenerative
diseases confirmed by diagnostic imaging
CSF exam
DNA testing or on PM
what are idiopathic epileptic seizures caused by?
unknown, what idiopathic means
may be a genetic link
no structural cause
what are the 4 stages of a seizure
prodrome
aura/pre-ictus
ictus or seizure
post-ictus
what is status epilepticus
SE = neurological emergency with a mortality of up to 25%
continuous epileptic seizure activity lasting longer than 5 minutes or as 2+ seizures with incomplete recovery of consciousness interictally
or continuous seizure activity for longer than 30 minutes
how do we treat seizures?
caused by an imbalance of inhibitory and excitatory activity in the brain
antiepileptic/anticonvulsive drugs restore the balance by facilitating inhibitory activity
how does seizure treatment work?
altering intrinsic membrane potentials (predominantly Na+ channels)
increasing inhibitory transmitter function, primarily in the GABA system
decreasing excitatory transmitter function, primarily glutamate system
what are the main, licensed drugs used for seizure treaments?
Benzodiazepines (short term use)
Barbiturates (long term use)
Imepetoin
KBr
primarily affect the GABAA receptor for Cl-
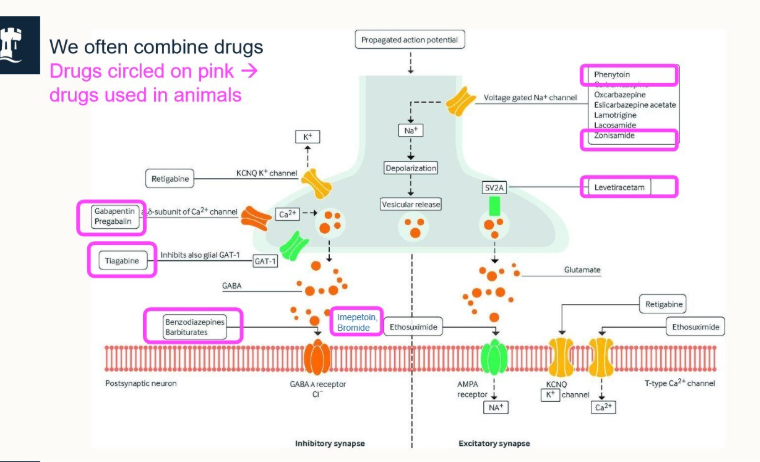
what is a simplification of how the seizure treatment works?
membranes are polarised by active transport and passage of electrolytes
seizures involve uncontrolled depolarisation of nerve cells and networks
anticonvulsants act by altering passage of electrolytes across membranes to produce hyperpolarisation
hyperpolarised membranes are harder to depolarise so seizure activity stops
what are we aiming to do with giving treatment for seizures?
control the seizures
reduce their frequency as much as possible
what do we need to consider when giving seizure drugs and how do we determine the dosage that needs to be given?
need to consider dosage - what will be effective? is it in the safe zone?
give a starting dosage for 2 weeks
ask the owner on review about any clinical signs e.g. barbiturates make pets very sleepy
assess on whether to make the dosage higher or lower
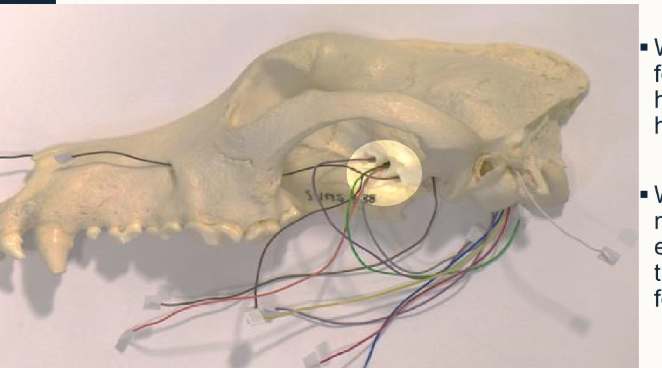
What foraminae are highlighted here and which nerves emerge from them?
Top to bottom:
Optic canal - CN II
Orbital fissure: Opthalmic division of CN V and nerves to extrinsic muscles of the eyeball
Rostral alar foramen/round foramen - passage of the maxillary division of CN V
what is different about the orbital fissure in pigs and small ruminants?
it’s fused with the round foramen to form the foramen orbitorotundum
name the 12 cranial nerves
olfactory
occipital
occulomotor
trochlear
trigeminal
aducens
facial
vestibulocochlear
glassopharyngeal
vagus
accessory
hypoglossal
what are the 3 parts of the trigeminal nerve
V1 = opthalmic branch
V2 = maxillary branch
V3 = mandibular branch
where does the olfactory nerve exit the skull?
cribiform plate (nerve travels down cerebrum to olfactory bulb through cribiform plate to voreronasal organ)
where does CN II leave the skull?
optic foramen/canal
where does CN III leave the skull?
orbital fissure
where does CN IV leave the skull?
orbital fissure
where do the V1, V2 and V3 branches of CN V leave the skull?
V1 - orbital fissure
V2 - rostral fissure
V3 - oval foramen
where does CN VI leave the skull?
orbital fissure
where does CN VII leave the skull
srylomastoid foramen
facial canal
where does CN VIII leave the skull?
passes through internal acoutsic meatus (with CN VII) from the medulla oblongata to the vestibulocochlear organ in the temporal bone
where does CN IX leave the skull?
jugular foramen
where does CN X leave the skull?
jugular foramen
where does CN XI leave the skull?
jugular foramen and foramen magnum
where does CN XII leave the skull?
hypoglossal canal/foramen
which nerve arises from the pons?
CN V - trigeminal
what 4 nerves emerge from the orbital fissure?
oculomotor
trochlear
abducens
V1 of trigeminal
outline whether the cranial nerves are Sensory, motor or both
CN I - S
CN II - S
CN III - M
CN IV - M
CN V - B
CN VI - M
CN VII - B
CN VIII - S
CN IX - B
CN X - B
CN XI - M
CN XII - M
what could an owner confuse a seizure with and why?
convulsions
vestibular disease
stroke
all involve being unable to stand, head tilt/eyes rapidly moving back and forth and maybe ataxia
what is the difference between intra- and extra-cranial causes of seizures?
intra originate in the brain (structural and functional)
Extra have causes outside of the brain
How can we subdivide intra-cranial causes of seizures?
structural lesions (vascular, inflammatory/infectious, traumatic, congenital, neoplastic disease)
No lesion is present that’s primary (functional or idiopathic epilepsy)
what are features of extra-cranial seizures?
Metabolic:
multifocal neurological examination - neurological deficits to the forebrain and NMSK system
inter-ictal signs usually present - ‘good’ and ‘bad’ days
symmetrical neurological signs most commonly seen
Toxic seizures:
not usually recurrent, discrete time period
myoclonus and twitching are common features
often accompanied with GI signs
Anoxic:
associated cardiorespiratory signs
associated triggers leading to increased vagal tone
what are features of intracranial seizures?
Symptomatic:
altered mentation
blindness
relentless pacing/circling
loss of learned behaviour
Idiopathic:
onset b/w 6 months and 6 years of age
normal inter-ictally
recurrent seizures
breed predisposition
what are some diseases often mistaken for seizures?
syncope
weakness
movement disorders
narcolepsy
myokymia and neuromyotonia
myotonia
postural myoclonus (idiopathic head bobbing)
vestibular episode
what are some intracranial causes of seizures?
idiopathic epilepsy
congenital disease e.g. hydrocephalus/storage disease
infectious meningoencephalitis
non-infectious meningoencephalitis
trauma
CNS neoplasia
nutritional e.g. thiamine deficiency
Vascular e.g. cerebrovascular accident e.g. infarction or haemorrhage
what are some metabolic causes of extracranial diseases?
Metabolic:
hypoglycamia
hypocalcaemia
hyoernatremia
hepatic encephalopathy
uremia
hyperlipoproteinemia
hypertensive encephalopathy
phlycythemia
what are some toxicity causes of extracranial causes of seizures?
lead toxicity
metaldehyde poisoning
ethylene glycol poisoning
strychnine toxicity
choclate
alpha-chloralose
chlorinated hydrocarbons
xylitol
what is an anoxic cause of extracranial seizures?
hypoxia e.g. cardiac or respiratory insufficiency
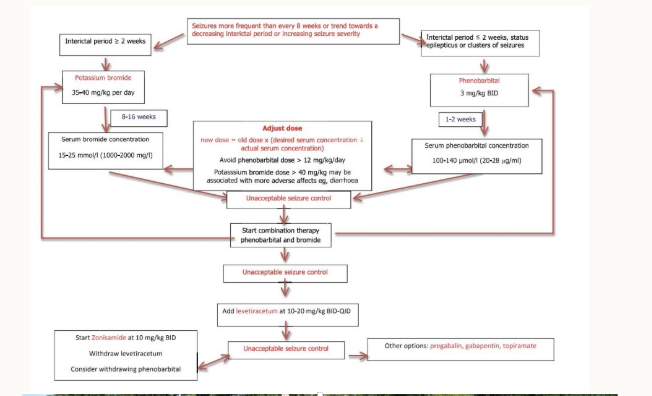
Outline the:
a) mechanism of action
b) elimination of half-life
c) approximate time to steady state
d) whether it’s used as a first/second treatment
of Phenoarbital
Acts on GABA receptors which increase synaptic inhibition - is a barbiturate
40-90 hours given every 12 hours
7-10 hours
first line treatment
Outline the:
a) mechanism of action
b) elimination of half-life
c) approximate time to steady state
d) whether it’s used as a first/second treatment
of KBr
acts on neuronal Cl- channels, causing hyperpolarisation of neuronal membranes, raising seizure threshold
24 days in dogs, 10 days in cats
up to 4 months in dogs, 6 weeks in cats
both
Outline the:
a) mechanism of action
b) elimination of half-life
c) approximate time to steady state
d) whether it’s used as a first/second treatment
Imepitoin
Activates receptors for GABA, increases GABA effects. Blocks some Ca2+ channels, less Ca2+ moves into the cell, preventing nerve impulses from being transmitted, thus reducing seizures
1.5-2hours
3 hours to reach a steady state
2nd - do not use as a 1st treatment in cases of cluster seizures or status elipticus.
what are the 3 groups of cranial nerves
special senses (CN I, II, VIII)
Innervation of head muscles (CN III, IV, VI, XII)
Innervation of structures originating from brachial arches (CN V, VII, IX, X, XI)
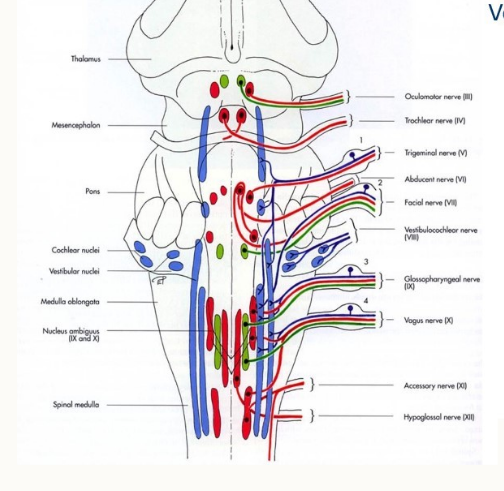
Name the nuclei of the cranial nerves
mesencephalon, midbrain
metencephalon, cerebellum and pons
myelencephalon (medulla oblongata)
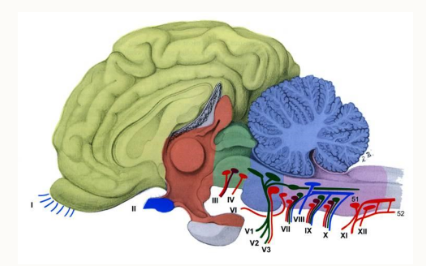
How are the nuclei arranged?
in uniformed locations
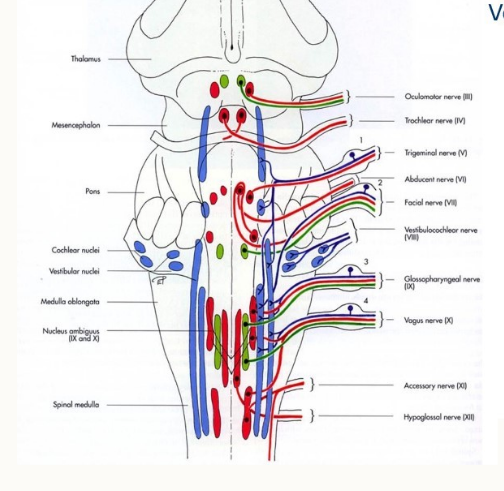
how do the cranial nerves egress?
passage through foramina of the skull
individually or in groups
Outline olfaction
olfaction and gustation
chemical senses
chemical substances stimulate special sensory cells and generate an action potential
impulse is transported via sensory afferent fibres to the brain
these fibres run in cranial nerves
Outline the sense of smell
olfactory organ is particularly well developed in dogs (200 more time sensitive than in humans)
important for orientation in the environment
olfactory mucous membrane in the nasal fundus = olfactory region
covered in olfactory epithelium
contains olfactory neurosensory cells
dog is macrosmatic
has a large olfactory region, 15-20 x larger than in humans
how do the olfactory nerves go from the nose to the brain?
through the ethmoid bone
in the nasal cavity we have scrolls of bone called turbinates which are attached to the ethmoid bone, therefore theese are called the ethmoid turbinates
what is contained in the olfactory epithleium
cilia
olfactory neurosensory cells
bulbs of dendrites → dendrites → axon (olfactory fibre)
supporting cells
basal cells
what is unique about olfactory cells?
continuously replaced by division of basal cells
live only 30-60 days
nerve cells are replaced regularly (even in adults)
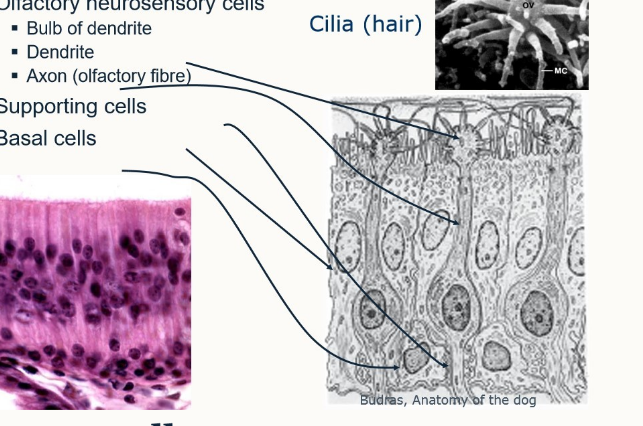
what kind of cells are olfactory cells
primary sensory cells
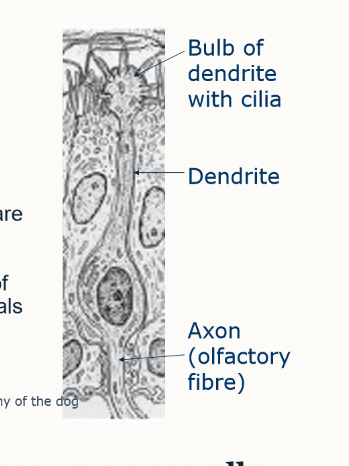
what is the passage of the olfactory fibres through the ethmoid bone known as?
cribriform plate
what is the key information for olfactory nerve - CN I?
Enters olfactory bulb
sensory nerves
special visceral afferent fibres
composed of many fibres = processes of olfactory cells of olfactory epithelium (receptor cells)
fibre bundles = olfactory filaments passes through cribriform plate
surrounding meningeal sheets include subarachnoideal space - potential routes for infection
where is the brain region for CN I?
cerebrum
what is the funciton (and functinal component) of CN I?
smell (SVA)
what form of clinical exam can we use to detect problems with CN I?
only owner’s observation - it’s very complex to test
what is an example of clinical signs seen after injury of CN I?
anosmia - loss of smell
outline the key information of the CN II
enters into the diencephalon
sensory nerve
special somatic afferent fibres
brain tract b/w retina (receptor) and diencephalon (origin)
in the optic chiasm, the fibres decussate
passes through the optic canal
what is special about the optic nerve?
myelinated by oligodendrocytes not schwann cells
this makes it involved in the CNS not the PNS
what is the optic chiasm
where the optic nerves cross over and joint together before entering the optic canal
not all nerves decussate
some of the left nerves go into the left forebrain, some cross over and enter the right forebrain (same on other side)
outline the pathway of conscious vision
eye
optic nerve
optic chiasm
optic nerve
lateral geniculate nucleus
occipital cortex (prosencephalon)
outline the optic pathway of the pupillary light response - mesencephalon one
optic nerve → optic chiasm → optic nerve → lateral geniculate nucleus
optic nerve → optic chiasm → optic nerve → prectal nucleus (bypasses lateral geniculate) → parasympathetic nucleus III → CN III
outline the optic pathway of the menace response
optic nerve → optic chiasm → optic nerve → lateral geniculate nucleus → occipital cortex → motor cortex → parasympathetic nucleus III → pontine nucleus (in cerebellar cortex) → motor nucleus 3 → CN VII → blink
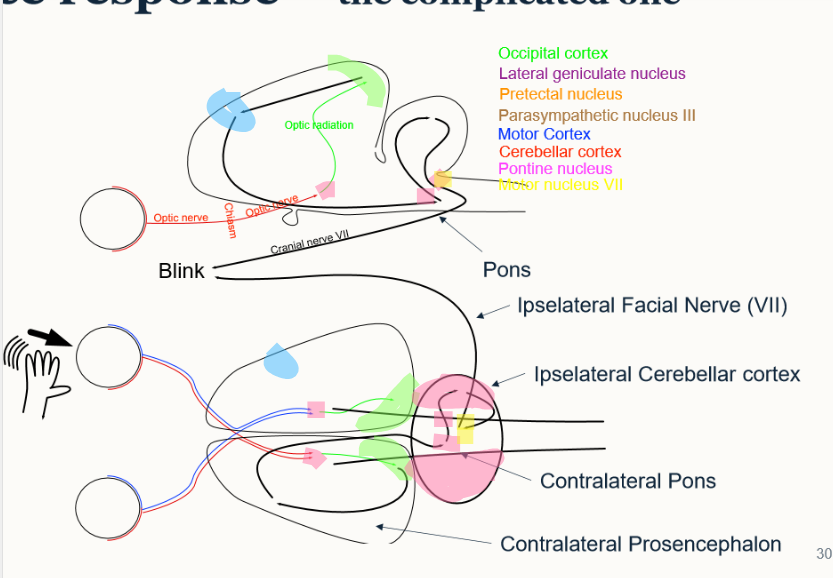
what is meant by peripheral blindness - what is affected?
absent vision without PLR
affected:
retina
pre chiasmal optic nerve
post chiasmal optic nerve
what is meant by central blindness and what is affected?
absent vison with PLR
affected:
lateral geniculate nucleus
occipital cortex
summarise CN II: name and number, brain region, function (functional components), clinical exam and clinical signs seen after injury
optic - II
diencephalon
vision (SSA)
menace response
anopsia - loss of vision
outline CN VIII
2 components - vestibular nerve (balance) and cochlear nerve (hearing)
sensory nerve
SSA
medulla oblongata
passes through internal acoustic meatus into petrosal bone
special sense of balance and hearing
what is the topographical course for VIII
enters petrosal bone through internal acoustic meatus with VII
summarise CN VIII: name and number, brain region, function (functional components), clinical exam and clinical signs seen after injury
vestibulocochlear nerve - VIII
medulla
balance and hearing (SSA)
hearing - horizontal and vertical eye movement
defness, head tilt (constant), nystagmus
outline CN III
ventral midbrain
motor nerve
GSE - general somatic efferent
6 muscles
4 go to the eyeball and to the levatory of upper eyelid: dorsal rectus, medial rectus, ventral rectus, ventral oblique
outline CN III
visceral efferent (PS)
pre-ganglionic from PS nucleus - midbrain
passes through orbital fissure
synapses in ciliary ganglion
M. spincter pupillae and M. ciliaris
what is anisocoria and what kind of issue is it?
a clinical representation of different sized pupils
autonomic issue
How can we identify aniscoria and what 2 kinds are there
imbalance between sympathetic and parasympathetic supply
sympathetic dilates - absence leads to a small pupil that won’t dilate in darkness (horner’s syndrome)
parasympathetic constricts - absence leads to dilated non-responsive pupil
How is the iris controlled?
parasympathetic - craniosacral → constriction
sympathetic - thoracolumbar → dilation
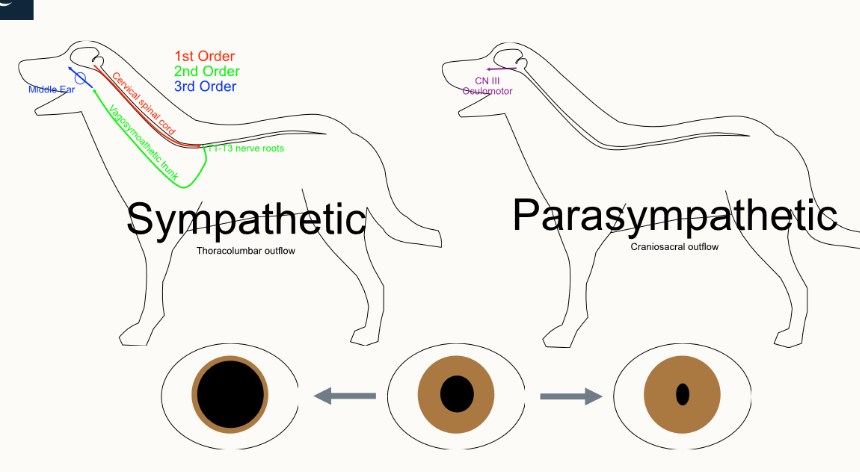
name 4 symptoms of Horner’s syndrome
meiosis
enophthalmus
ptosis
3rd eyelid potrusion