AP Chemistry Unit 5 Chemical Kinetics
1/26
Earn XP
Description and Tags
NOT COMPLETED
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
difference between thermodynamics and kinetics
thermodynamics - does a reaction take place?
kinetics - how fast does a reaction proceed?
reaction rate
change in concentration of a reactant or a product with time
instantaneous rate
rate for specific instance in time
rate law
expresses the relationship of the rate of a reaction to the rate constant and the concentrations of the reactants raised to some powers
the rate law
k[A]^x[B]^y
reaction is xth order in A
reaction is yth order in B
reaction is (x+y)th order overall
true or false: rate laws are always determined experimentally
true
true or false: reaction order is not always defined in terms of reactant concentrations.
false
true or false: the order of a reaction is directly related to the stoichiometric coefficient of the reactant in the balanced chemical equation
false
half life definition
time required for concentration of a reactant to decrease to half of its initial concentration
reaction rate formulas
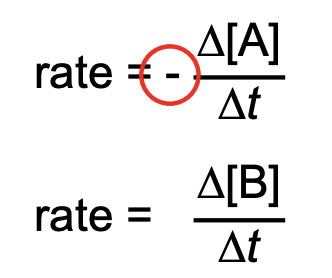
rate law for zero order reaction
rate = k
rate law for first order reaction
rate = k [A]
rate law for second order reaction
rate = k [A]²
concentration-time equation for zero order reaction
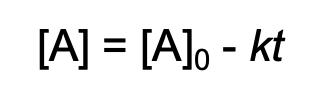
concentration-time equation for first order reaction
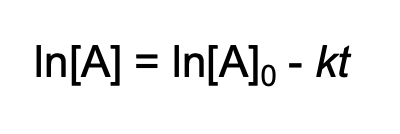
concentration-time equation for second order reaction
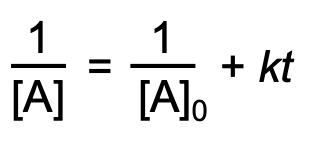
half-life for zero order reaction
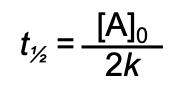
half-life for first order reaction
or 0.693/k
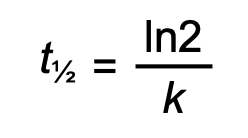
half-life for second order reaction
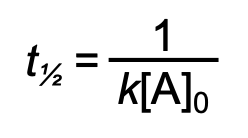
activation energy
minimum amount of energy required to initiate a chemical reaction
endothermic reaction
more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the products
exothermic reaction
more energy is released when the bonds are formed in the products than is used to break the bonds in the reactants
arrhenius equation
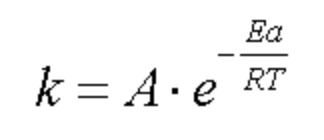
what is the arrhenius equation used for
to determine the effect of a change of temperature on the rate constant
units for rate
(ms)^-1
units for k in first order reaction
1/s
units for k in second order reaction
1/ms