Unit 8 - the crystal structure of solids
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
What is a crystalline material?
the atoms are situated in repeating or periodic array over large atomic distances
usually metals and soilds
What are non crystalline amorphous solids?
do not have a regular structure, no long-range atomic order
glasses and polymers
rapid cooling favours this as there is little time for the ordering process
what is a lattice?
three dimensional array of points coinciding with sphere centres
What is a unit cell?
the basic structural unit of the crystal structure
not in amorphous structures
How many groups of bravais lattices are there?
7
what are the angles and side lengths in a cubic?
a=b=c
90
what are the angles and side lengths in a tetragonal
a=b =/c
90
what are the angles and side lengths in a orthorhombic?
a=/b=/c
90
what are the angles and side lengths in a monoclinic?
a=/b=/c
a and gamma 90
what are the angles and side lengths in a hexagonal
a=b=/c
alpha and beta 90, gamma 120
what are the angles and side lengths in a rhombohedral
a=b=c
alpha and beta 90
what are the angles and side lengths in a triclinic?
nothing equal
How many possible unit cells are there?
14 possible repetitive unit cells which causes the complete filing of a 3D space
Why do half of elemental solids have FCC or HCP packing?
efficient close packing and hence low energy
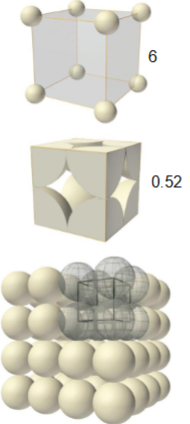
What is a simple cubic unit cell
Atomic packing factor - 0.52
coordination number - 6
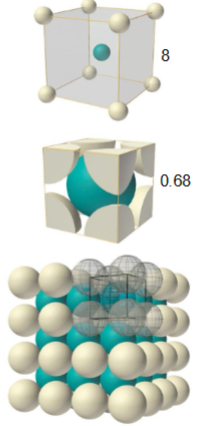
what is a body centered cubic unit cell?
atomic packing factor - 0.68
coordination number - 8
harder and less malleable metals, slip is harder
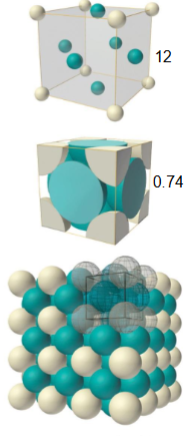
What is a face centred cubic unit cell?
atomic packing factor - 0.74
coordination number - 12
what is the atomic packing factor?
fraction of solid sphere volume in a unit cell assuming the hard sphere model.
fcc has 74% which is the maximum packing possible for spheres to have the same diameter
What is the hexagonal closed packed crystal structure
atomic packing factor - 0.74
coordination number 12
same as FCC same volume of atoms per unit cell the only difference is the stacking sequence
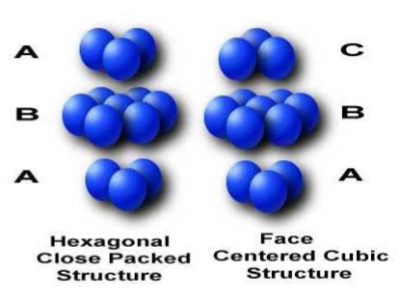
The HCP and FCC stacking sequence
FCC - ABCABC
HCP - ABAB
Why is graphite and diamond so different to each other?
graphite - soft, dark-coloured material
diamond - hard, transparent
the reason they are so different is the 2 different crystal structures
the higher the apf, easier deformation