Lecture 4: 🤍Necrosis and Apoptosis
1/32
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
What are the three hypobiotic processes?
Degeneration, Necrosis, Atrophy
Type of damaged cells and tissues → worse phys. functions, with focus on catabolic processes and structural changes in organs.
Differences between reversible and irreversible injury?
cell and organelle
reversible: generalized swelling
irreversible: incr. swelling, disruption of lysosomes
in plasma membranes:
reversible: blebbing
irreversible: disruption
Ribosomes - reversible injury cause detachment from ER
Nuclear chromatin:
reversible: clumping
Irreversible: profound changes
Mitochondria - Irreversible injury cause presence of large amorphous densities in swollen mitochondria.
Cellular membranes - Irreversible injury causes disruption + nuclear changes.
Myelin figures:
Reversible: Myelin figures/laminated structures come from damaged membranes
Irreversible. more pronounced in irreversible damaged cells.
Name two types of cell death.
Necrosis (Oncosis): Cell swelling, oncotic cell death
Apoptosis: Cell shrinkage
Definition of Apoptosis
Form of programmed cell death. Which can happen in two ways:
Physiological: naturally occurring, helping with organ development + size maintenance.
Toxic: triggered by external factors, chemicals, pathogens, lack of O2.
Intracellular suicide - Apoptosis is like a cell`s built in self-destruction button.
In which two phases do the Apoptosis work?
Initiation Phase: caspases (enzymes) become active.
Execution Phase: caspases cause cell death.
Process is faster than necrosis, completing in about 6h.
What are the initial ultrastructural changes in necrosis vs. Apoptosis?
Necrosis:
Cytoplasmic blebs, digestion and leakage of cellular components
Apoptosis:
Initial changes: nuclear chromatin condensation and fragmentation
further events: cytoplasmic budding and phagocytosis of the extruded apoptotic bodies
The differences in Apoptosis and necrosis
affected cells?
chromatin?
DNA fragmentation?
Cytoplasm and cell volume will?
organelles will?
cell integrity?
cell contents?
affected cells
Apoptosis: scattered individual cells
Necrosis: massive + contiguous cells
chromatin
Apoptosis: marginates as large crescent aggregates
Necrosis: Marginates as small aggregates
DNA fragmentation
Apoptosis: Ladder pattern
Necrosis: smear pattern
Cytoplasm and cell volume will:
Apoptosis: decrease
Necrosis: Increase
organelles will:
Apoptosis: Retain integrity
Necrosis: swell (mitochondria, ER)
cell integrity:
Apoptosis: breaks into small fragments
necrosis: rupture
cell contents:
Apoptosis: cell fragments are phagocytized
Necrosis: content is released
Is inflammation present in apoptosis or necrosis?
Apoptosis: No inflammation
Necrosis: Extensive inflammation.
what is the difference between Necrosis and Necrobiosis?
Necrosis: Intravital (occurs in a living organism) death of a cell and represents the final, irreversible state. (24-48h)
Necrobiosis: the process of degeneration and cell death. Morphological changes are visible after some time due to enzymatic effects. (6-12h)
Describes the process that leads to necrosis.
List 5 factors that can cause cell damage leading to necrosis.
Poisons
Lack of Blood Supply
Lack of Nerve Supply
Pressure
Mechanical and Thermal Injuries (burns ex.)
Describe the macroscopic appearance of necrotic tissue.
Paler than living tissue, little tensile strength, hemolysis of blood cells, and slight inflammatory zone.
Name nuclear changes - necrosis
Pyknosis
Hyperchromatosis
Karyorrhexis
Karyolysis
Define pyknosis.
Necrobiotic change on nuclei.
Nucleus decreases in size, becomes rounder, chromatin condenses, appears dark and homogenous.
One of the earlier changes.
Define hyperchromatosis
Necrobiotic change on nuclei.
Chromatin concentrates around the edge (nuclear membrane) of the nucleus, making the centre look paler.
Define karyorrhexis.
Necrobiotic change on nuclei.
The nuclear membrane breaks apart, and the chromatin scatters into small, dark stained clumps (aggregates).
Define karyolysis.
Necrobiotic change of nuclei.
The nuclear material dissolves and fades away.
nucleus is not seen, when this is complete, but the term only refer to those incomplete stages when the nucleus appears as a hollow sphere with only the nuclear membrane remaining.
Describe acidophilia of cytoplasm.
This occurs because intracellular proteins denature, ribosomes disappear → increased eosin binding, more visible mitochondria (appearing as eosinophilic granules)
Eosin is an acidic stain → red, so cytoplasm stains a deeper red than usual.
What happens during cytoplasmolysis?
Dissolution of cytoplasm and cytoplasmic membrane.
With the changes during the dying process → cytoplasm becomes less and less visible → disappearing completely.
In tissue with low fluid → opaque cytoplasm, homogenous to glassy, small granules.
Changes on extracellular matrix during necrosis can be?
Change of staining
fibrillar mass dissolves to amorphous state: the organized, fibrous structure of matrix become disorganized and in a shapeless state.
Necrotic tissue turns into necrotic debris of protein and fatty nubs
Name two main types of necrosis.
Coagulative Necrosis
Colliquative (Liquefactive) Necrosis
Describe coagulative necrosis.
Results from denaturation of cellular proteins, leading to a firm mass of necrotic cells. Occurs in parenchymatous organs, due to high protein content.
Macro: firm consistency, greyish-whitish to greyish-yellowish, dry, cloudy.
Micro: homogenous and eosinophilic cytoplasm
Subtypes:
caseous necrosis
Zenker necrosis
Describe liquefactive (colliquative) necrosis.
Type of cell death - Rapid liquefaction (breakdown + dissolution) of dead cells due to lysosomal enzymes (hydrolases) released from neutrophils and autolytic digestion.
Abscesses: When liquefactive nercrosis happens and the liquid is represented by pus → considered to be an abscess.
Where is liquefactive necrosis commonly observed in the central nervous system, and what is it called?
It is called Malacia. Breakdown of myelin, leading to brain softening (encephalomalacia) and myelomalacia in spinal cord).
Describe Zenker's necrosis.
Occurs in striated muscle with coagulation of sarcoplasm proteins.
Macro: Muscle becomes white or pale, shiny, and swollen.
Micro: swollen fibers, homogenous and hyaline, acidophilic sarcoplasm, lack of cross striation in myofibrils + small and dark nuclei.
cause: nutritional and toxic myopathies.
Describe caseation.
Typical of tuberculous lesions, appearing as friable, cheesy, amorphous material.
Resembles cottage cheese, due to mix of degenerative tissue protein and fat coming from the lipid capsule of the organism.
What causes hemorrhagic necrosis?
Necrotic tissue congested with blood, often due to blockage of venous drainage → blood stasis + leakage such as in cases of volvulus.
Name two types of fat necrosis.
Occurs in the abdominal cavity or subcutaneous tissue - in adipose (fatty) tissue.
Traumatic: rupture of fat cells → tissue reaction → fibroblastic scar tissue formation.
Enzymatic (pancreatic): lipases split neutral fats → FA + glycerol, where the FA combine with alkalis to form chalky precipitates (saponification).
Abdominal: large masses of necrotic fat in omentum, mesentery + retro-peritoneal area. In cattle.
List 5 outcomes of necrosis.
Liquefaction and removal of fluid
Liquefaction and cyst formation
Liquefaction with abscess formation
Encapsulation without liquefaction
Desquamation or sloughing
gangrene, calcification, atrophy, regeneration
Define sequestration in the context of necrosis.
When a necrotic area becomes walled off or demarcated from the surrounding healthy tissue. This happens because mediators released from the necrotic tissue cause neutrophils + other cells to accumulate at the margin.
Define gangrene.
Necrosis modified by secondary changes. Occurs in skin, lung, intestine and mammary gland.
Describe dry gangrene.
Caused by slow reduction of blood flow in arteries. The tissues don`t get enough blood over a long period. Results in the tissues drying out and shriveling up.
Tissues are dry, shrunken, cold, no pulse, brown to black.
Describe moist (wet) gangrene.
Caused when there is sudden stop of blood flow to tissue that is already full of blood. This can happen due to burns, freezing, or injuries.
The tissue will die → breeding ground for bacteria → putrefaction (rotting).
Tissue is swollen, soft, moist, reddish-brown, gas bubbles and a bad smell.
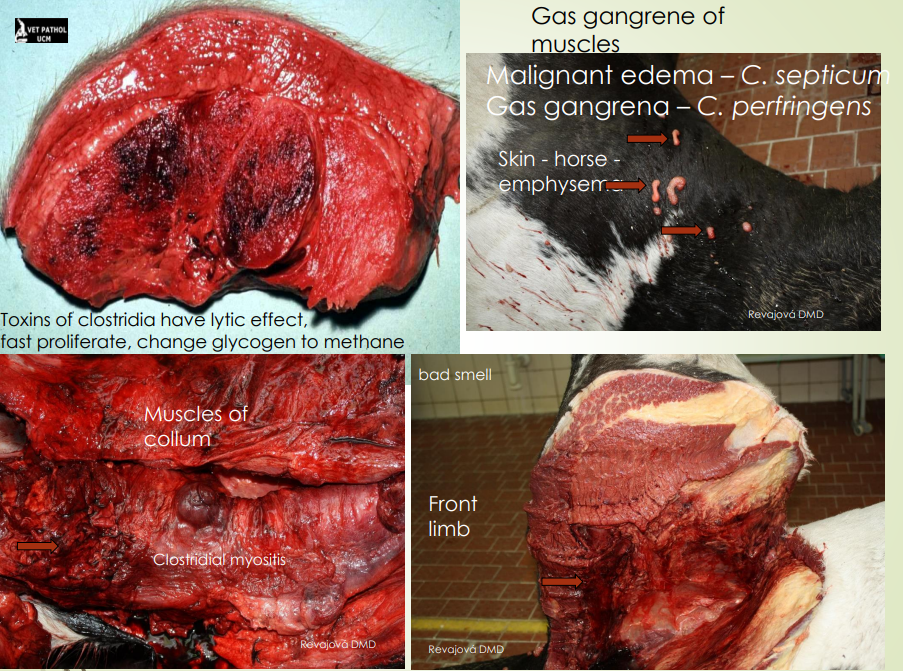
Describe gas gangrene.
Caused by a severe infection of damaged tissues by anaerobic bacteria, especially Clostridium species. This happens usually in dirty, deep wounds.
The bacteria produce toxins → destroying tissue + releasing gas.
The muscles and tissues will fill with gas, serohemorrhagic exudate is present (with blood), affected area turns dark red to black.