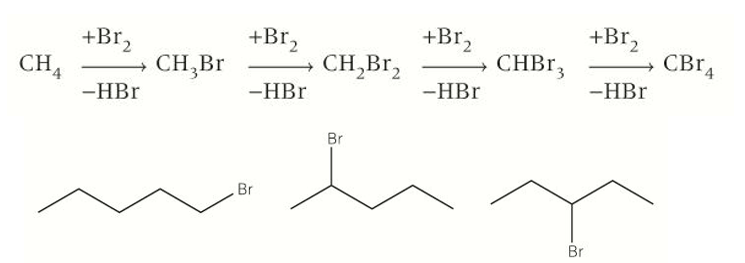
Reactions of alkanes (Radical substitution)
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
Three basic steps of radical substitution
Initiation
Propagation
Termination
It is uncontrollable and starts with a flash of light, gives us a mixture of products.
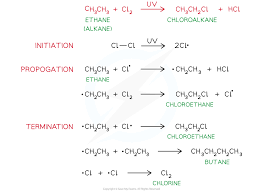
Initiation
Homolytic fission which forms radicals, and must be under UV light. Radicals react quickly with surrounding molecules.

Propagation
These reactions will repeat millions of times.
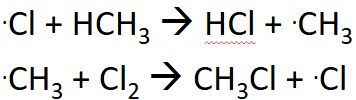
Termination
This is the death of radicals, they combine to form stable molecules happens when radicals have finished with reacting with other molecules.

Limitations of radical substitution
More than one halogen can substitute into a molecule, creating a mixture of products and something we don’t want.
Substitution can occur at different positions along the chain giving us an unwanted product
Further substitution can occur past our wanted product