biochem exam 3
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
71 Terms
Energy poor end products
h20, co2, nh3
cell macromolecules
proteins, polysaccarides, lipids, nucleic acid
precursor molecules
amino acid, sugars, fatty acid, nitrogenous base
G > 0 endergonic (positive)
energy absorbed, reaction not spontaneous, requires input of energy, more products than reactants
G < 0 exergonic (negative)
energy is released, reaction is spontaneous, more reactants than products
electron carriers
NAD+ and FAD are electron carriers that capture electrons from oxidized fuel.
NAD+ picks up electrons as H:- (hydride anion), whereas FAD+ can pick up free radicals (H●)
Tissues that may use anaerobic glycolysis
Red blood cells – exclusively
White blood cells
Kidney medulla
Eye tissues
Skeletal muscle
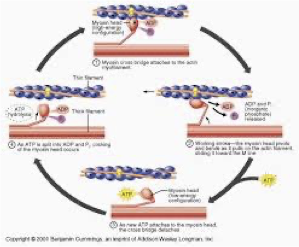
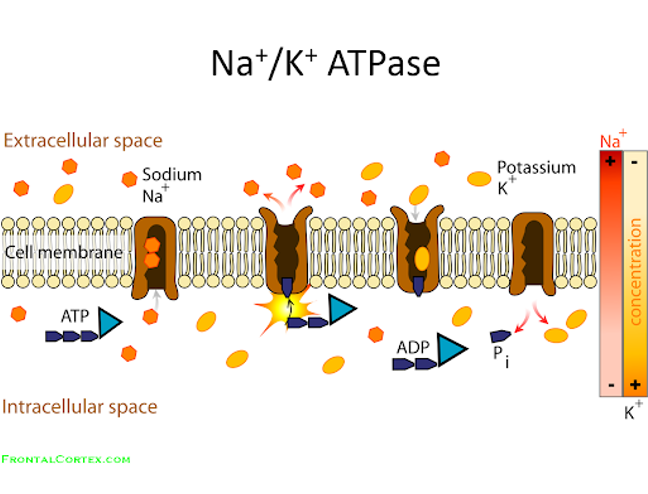
which uses most ATP
Protein synthesis and Na+K+ ATPase (maintain membrane potentials) use most ATP
Gluconeogeneis and urea synthesis occur in the ___
liver
Synthesis
forms covalent bonds
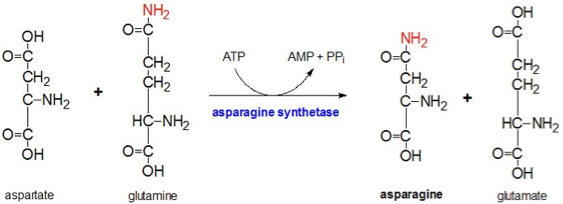
Synthesis: asparagine synthetase
ATP is hydrolyzed to provide the driving force for asparagine synthesis, but none of its atoms are incorporated into asparagine or glutamate. ATP here is purely an energy donor, not a substrate incorporated into the product.
irreversible, rate-limiting, committed step of glycolysis, where PFK-1 uses ATP to add a second phosphate to fructose-6-phosphate, producing fructose-1,6-bisphosphate and ensuring glycolysis continues toward energy production.
Creatine phosphate
used when you need to contract muscle quickly
Amount of energy captured from fuel depends on oxidation state
C-O vs C-C, C-H
more C-O = more oxidized
oxidation image
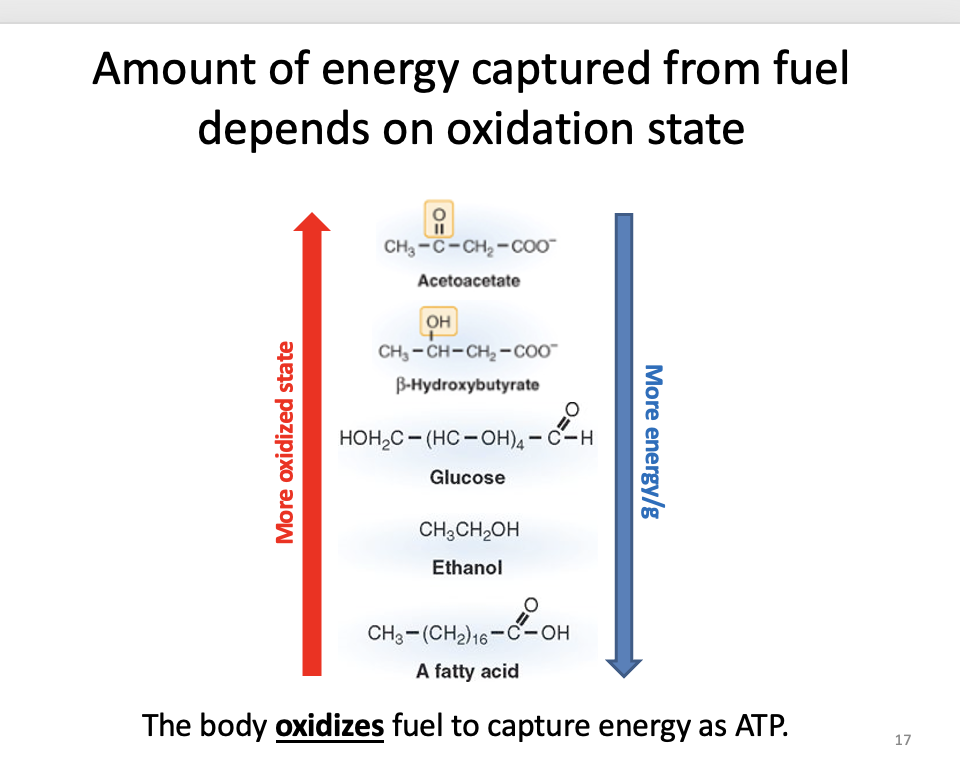
which is least oxidized and has most energy
fatty acid
Oxidizing fuel = losing electrons (LEO)
Niacin is a precursor for NAD+
Riboflavin is a precursor for FAD synthesis
NAD+ and FAD are essential for aerobic metabolism
•Collect e- s from catabolism of CH2O, proteins, lipids
•Found in dehydrogenase enzyme reactions
•NAD+, FAD regenerated when transfer electrons
•Act as “shuttles” to carry e- s
–From catalysis to the mitochondria
–Eventually added to O2 to form H2O
dehydrogenase enzyme
redox reaction reducing NAD+ to NADH
Tissues that may use anaerobic glycolysis:
Red blood cells – exclusively bc no mitochondira
White blood cells
Kidney medulla
Eye tissues
Skeletal muscle
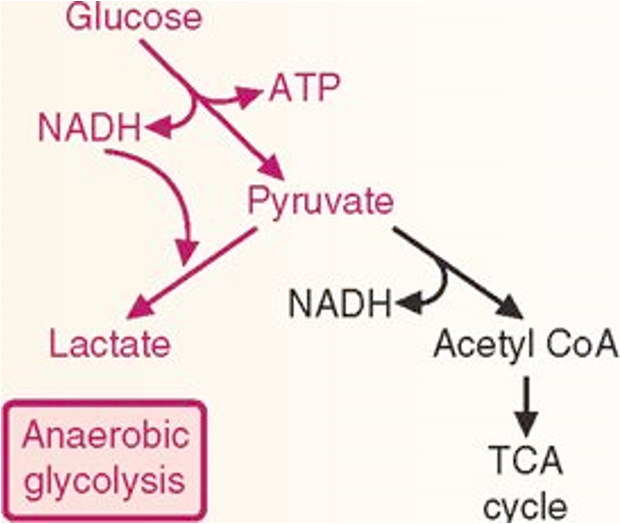
Aerobic vs. anaerobic metabolism
1. Glucose
Glucose enters glycolysis.
Yields:
2 ATP (net gain)
2 NADH
2 Pyruvate
2. Anaerobic Pathway
anaerobic conditions
Pyruvate is reduced to lactate by lactate dehydrogenase.
This reaction oxidizes NADH back to NAD⁺.
NAD⁺ is essential to keep glycolysis running (otherwise glycolysis would stop because NAD⁺ is required at the glyceraldehyde-3-phosphate dehydrogenase step).
Key role of lactate production: regenerate NAD⁺ so glycolysis can continue to make ATP in low-oxygen conditions (like exercising muscle).
3. Aerobic Pathway
In the presence of oxygen:
Pyruvate enters mitochondria.
Converted to Acetyl-CoA (via pyruvate dehydrogenase).
Acetyl-CoA enters the TCA cycle, producing 6 NADH and 2 FADH₂ and 2 GTP
These feed into the electron transport chain (ETC) → 28 ATP produced by oxidative phosphorylation.
↑ thyroid hormone levels which accelerate basal metabolic processes. –How does this relate to ATP? –Why did he lose weight?
increase in ATP consumption b/c increase ATP demand
lost weight bc he was using stored fuels
–What happened to her heart’s ATP production during the MI?
–What consequences do you think this will have?
decreased ATP produced
dependent on anaerobic glycolysis to keep ATP going so increase in lactic acid bc heart muscles are regenerating NAD+ to continue glycolysis and there will be a decrease in Na+/K+ ATPase
mechanical
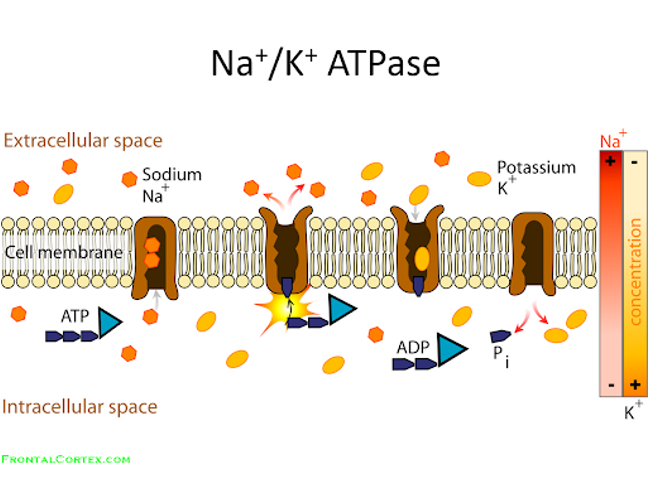
transport bc moving solutes against conc. gradient
bichemical
isocitrate is ____ to ketoglutarate
oxidized
pyruvate to lactate
oxidized
b-hydroxybutrate to acetoacetate
oxidized
pyruvate to lactate
reduced
testosterone to estradiol
reduced
Glycolysis invests ATP to generate energy
Phase I: Preparative (Energy Investment) Phase
6 C
2 atp used
Phase II: ATP-Generating (Payoff) Phase
2 C, 4 atp total, net atp = 2
Glycolysis: Investment Phase
AKA preparative phase
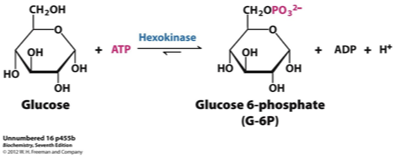
Step 1: Glucose → Glucose-6-phosphate
Enzyme: Hexokinase (or glucokinase in the liver).
What happens: ATP donates a phosphate → glucose becomes glucose-6-phosphate (G6P).
Purpose: Traps glucose in the cell and prepares it for further breakdown.
Step 2: Glucose-6-phosphate → Fructose-6-phosphate
Enzyme: Phosphoglucose isomerase.
What happens: G6P (a 6-membered ring) is rearranged into fructose-6-phosphate (F6P) (a 5-membered ring).
Purpose: Sets up the molecule for a second phosphorylation step.
Step 3: Fructose-6-phosphate → Fructose-1,6-bisphosphate
Enzyme: Phosphofructokinase-1 (PFK-1).
What happens: ATP donates another phosphate → product is fructose-1,6-bisphosphate (F-1,6-BP).
Purpose: This is the committed, rate-limiting step of glycolysis (once here, glucose must continue through glycolysis).
Step 4: Splitting Step
Enzyme: Aldolase.
What happens: Fructose-1,6-bisphosphate is split into two 3-carbon sugars:
Dihydroxyacetone phosphate (DHAP)
Glyceraldehyde-3-phosphate (G3P)
These two are interconvertible via the enzyme triose phosphate isomerase.
Only G3P continues directly into the payoff phase of glycolysis, but DHAP can be converted into G3P so both carbons are used.
Glycolysis: Pay-off Phase
AKA ATP-generating phase
Starting Molecule
Glyceraldehyde-3-phosphate (G3P) (produced from splitting fructose-1,6-bisphosphate).
Each glucose gives 2 G3P molecules, so all steps here happen twice per glucose.
Step 1: G3P → 1,3-Bisphosphoglycerate (1,3-BPG)
Enzyme: Glyceraldehyde-3-phosphate dehydrogenase.
What happens:
G3P is oxidized, NAD⁺ is reduced to NADH.
An inorganic phosphate is added, forming 1,3-BPG, a high-energy intermediate.
Yield: 2 NADH per glucose.
Step 2: 1,3-BPG → 3-Phosphoglycerate (3PG)
Enzyme: Phosphoglycerate kinase.
What happens:
1,3-BPG donates a phosphate to ADP → makes ATP.
Yield: 2 ATP per glucose (substrate-level phosphorylation).
Step 3: 3PG → 2-Phosphoglycerate (2PG)
Enzyme: Phosphoglycerate mutase.
What happens: Rearrangement of phosphate group from C3 to C2.
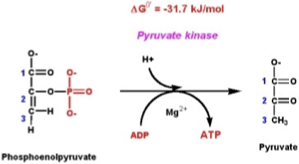
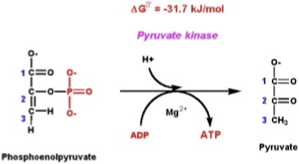
Step 4: 2PG → Phosphoenolpyruvate (PEP)
Enzyme: Enolase.
What happens: Removal of water → forms PEP, a very high-energy compound.
Step 5: PEP → Pyruvate
Enzyme: Pyruvate kinase.
What happens:
PEP donates its phosphate to ADP → forms another ATP.
End product = Pyruvate.
Yield: 2 ATP per glucose (substrate-level phosphorylation).
Net Yield (Payoff Phase, per glucose)
4 ATP produced (2 from Step 2 + 2 from Step 5).
2 NADH produced (from Step 1).
End product: 2 Pyruvate.
Free energy changes associated with steps in glycolysis
•Large ΔG is physiologically irreversible; these are regulated steps.
•Gluconeogenesis is not simply a reversal of glycolysis.
•Which reactions do you think are unique to glycolysis?
hexokinase
phosphofructokinase (PFK)
pyruvate kinase
Glycolysis produces pyruvate and NADH for aerobic metabolism
All cells carry out glycolysis in cytosol
Malate shuttle delivers electrons (from NADH) to inner mitochondrial space
Step 1: In the Cytosol
Glycolysis generates 2 NADH in the cytosol.
NADH cannot cross the mitochondrial membrane.
Instead:
Oxaloacetate is reduced to malate by malate dehydrogenase, using electrons from NADH.
This regenerates NAD⁺ in the cytosol so glycolysis can continue.
Malate can cross the inner mitochondrial membrane.
Step 2: In the Mitochondrial Matrix
Once inside, malate is reoxidized back to oxaloacetate, producing NADH in the mitochondrial matrix.
This mitochondrial NADH can feed electrons into the electron transport chain to generate ATP.
Step 3: Oxaloacetate Recycling
Oxaloacetate cannot cross the mitochondrial membrane directly.
To recycle it:
Oxaloacetate undergoes a transamination (TA) reaction with glutamate, forming aspartate + α-ketoglutarate (α-KG).
Aspartate and α-KG can cross the membrane.
In the cytosol, the reaction is reversed to regenerate oxaloacetate + glutamate.
_____ is converted to ______ in absence of O2
Pyruvate is converted to lactate in absence of O2
And in cells without mitochondria (RBCs)
Aerobic glycolysis
Glycolysis (cytosol):
Glucose → 2 pyruvate.
Produces 2 ATP (net) and 2 NADH.
NADH Shuttles:
Cytosolic NADH can’t cross the mitochondrial membrane directly.
Electrons are transferred into the mitochondria via either the:
Malate–aspartate shuttle (efficient, regenerates NADH).
Glycerol-3-phosphate shuttle (less efficient, regenerates FADH₂).
Pyruvate in mitochondria:
Converted to Acetyl-CoA.
Enters the TCA cycle → generates NADH, FADH₂, GTP (ATP equivalent).
ETC & Oxidative Phosphorylation:
NADH and FADH₂ donate electrons to the electron transport chain.
Electrons flow to O₂ (final electron acceptor), producing H₂O.
Proton gradient powers ATP synthase → large ATP yield.
Anerobic glycolysis (lactate dehydrogenase)
Glycolysis (cytosol):
Same as aerobic: Glucose → 2 pyruvate.
Produces 2 ATP (net) and 2 NADH.
No oxygen available:
ETC cannot accept electrons because O₂ (final acceptor) is missing.
NADH builds up, and glycolysis would stop without NAD⁺ regeneration.
Lactate Production:
Pyruvate is reduced to lactate by lactate dehydrogenase.
This reaction oxidizes NADH → NAD⁺, allowing glycolysis to continue.
ATP yield per glucose (anaerobic): only 2 ATP.
why do cells form lactate in anaerobic glycolysis?
Lactate dehydrogenase converts pyruvate → lactate, and in the process oxidizes NADH → NAD⁺, keeping glycolysis going.
Why does NAD+ need to be regenerated?
Pyruvate is reduced → gains electrons from NADH → becomes lactate.
NADH is oxidized → loses electrons → regenerates NAD⁺.
The regenerated NAD⁺ goes back into glycolysis, enabling the G3P → 1,3-BPG step to continue.
The key role here: LDH restores NAD⁺ in the absence of oxygen.
Importance
Anaerobic conditions (no O₂): ETC cannot oxidize NADH → NADH builds up, NAD⁺ runs out.
LDH provides an alternate pathway to regenerate NAD⁺.
This keeps glycolysis running → ATP still produced (2 ATP per glucose).
Tissue Distribution
Skeletal muscle (fast-twitch): produces lactate during intense exercise when O₂ is limited. Drop in pH = muscle cramping
_____ plays a major role in removal of lactate and H+
Liver
Cori cycle
Step 1: Anaerobic Glycolysis (in RBCs)
RBCs and working muscle rely on glycolysis (either because they lack mitochondria, like RBCs, or oxygen is limited, like in intense exercise).
Glucose → 2 pyruvate → 2 lactate (via lactate dehydrogenase).
This process produces 2 ATP per glucose, enough for short-term energy.
Lactate is then released into the blood.
Step 2: Transport to Liver
Lactate travels through the bloodstream to the liver.
Step 3: Gluconeogenesis in the Liver
In the liver, lactate → pyruvate (via lactate dehydrogenase).
Pyruvate enters gluconeogenesis, which consumes 6 ATP equivalents to regenerate glucose.
This is an energy-expensive process, but it detoxifies lactate (prevents lactic acidosis) and provides fresh glucose for tissues.
Step 4: Glucose Exported Back to Blood
The newly made glucose is released into the blood.
It can be taken up again by RBCs or muscle to fuel glycolysis → continuing the cycle.
Why regulate glycolysis
Energy Balance
Avoid wasting glucose when ATP is plentiful.
Accelerate glycolysis when ATP demand is high.
Metabolic Flexibility
Glucose isn’t just for ATP — it’s also a precursor for biosynthesis (nucleotides, amino acids, fatty acids).
Pathway must adapt to cellular needs.
Tissue-Specific Roles
In muscle, glycolysis speeds up with exercise (energy).
In liver, glycolysis regulates blood glucose levels (fuel homeostasis).
Prevent Futile Cycles
Regulation ensures they don’t run simultaneously (which would just waste ATP).
•What steps are good targets for regulation?
irreversible steps with large negative ΔG
1. Hexokinase/Glucokinase
Reaction: Glucose → Glucose-6-phosphate.
Role: Traps glucose inside the cell.
Regulated by:
Product inhibition (G6P).
In the liver, glucokinase is regulated by insulin/glucagon
2. Phosphofructokinase-1 (PFK-1)
Reaction: Fructose-6-phosphate → Fructose-1,6-bisphosphate.
Role: Rate-limiting, committed step of glycolysis.
Regulated by:
Inhibitors: ATP, citrate (signal high energy).
Activators: AMP, ADP, fructose-2,6-bisphosphate (signal low energy/need for glucose breakdown).
3. Pyruvate Kinase
Reaction: Phosphoenolpyruvate (PEP) → Pyruvate.
Role: Final ATP-producing step.
Enzymes may be regulated by:
1.Substrate availability: promote enzyme catalyst step
2.[enzymes]: increase enzymes (gene expression) to increase glycolysis
3.Covalent modification of enzyme: phosphorylation of enzyme: phosphorylation of enzyme
4.Allosteric regulation
Molecules that can stimulate glycolysis
↑ [AMP], [ADP]
↑ [fructose-2,6-bisP]
↑ [fructose-1,6-bisP]
Molecules that can inhibit glycolysis
↑ [ATP] “We already have energy.”
↑ [citrate] used to make FA
↑ [glucose-6-P]
↑ [NADH]
↑ [acetyl-coA]
↑ [alanine] “We have enough pyruvate/building blocks.”
Regulation of glycolysis
1. Hexokinase (Glucose → Glucose-6-phosphate)
Inhibited by: Glucose-6-phosphate (product inhibition).
Logic: If glycolysis or glycogen synthesis downstream is backed up, hexokinase stops phosphorylating more glucose — prevents trapping unnecessary glucose in the cell.
2. Phosphofructokinase-1 (PFK-1)
The committed, rate-limiting step of glycolysis.
Activated by:
AMP, ADP → signal low energy.
Fructose-2,6-bisphosphate → powerful activator in the liver; ensures glycolysis is turned ON when blood glucose is high (insulin stimulates its production).
Inhibited by:
ATP → high energy charge, glycolysis not needed.
Citrate → signals TCA cycle is full, no need to make more acetyl-CoA.
the main control point for glycolysis.
3. Pyruvate Kinase (PEP → Pyruvate)
Activated by:
Fructose-1,6-bisphosphate (feed-forward activation: ensures if glycolysis has started, it finishes).
Inhibited by:
ATP → high energy, glycolysis not needed.
In the liver, glucagon inactivates pyruvate kinase (via phosphorylation) during fasting to spare glucose.
4. Pyruvate Dehydrogenase (PDH, Mitochondria)
Converts pyruvate → Acetyl-CoA (link between glycolysis and TCA cycle).
Activated by:
ADP → signals low energy.
Ca²⁺ → in muscle, signals contraction/energy demand.
Inhibited by:
NADH → high reducing equivalents, ETC backed up.
Acetyl-CoA → signals TCA cycle has enough input, don’t make more.
Glucose fates
1) . Glucose → Acetyl-CoA → CO₂
2). Glucose → Pentose Phosphate Pathway (PPP)
3). Glucose → Amino Sugars → Glycosaminoglycans
4. Glucose → Glycogen
5. Glucose → Lactate
6. Glucose ↔ Fructose / Lactose
Glucose and diabetes – cataracts
–Glucose increases rate of sorbitol, fructose formation
–Glucose & fructose increase nonenzymatic glycation of lens proteins (covalently attached)
•High rate of glucose uptake and glycolysis in cancer
–Initially due to lack of blood supply (prior to angiogenesis)
–Continues even in presence of functioning mitochondria (Warburg effect)
•Problems with the glycolytic enzymes are uncommon
pyruvate kinase deficiency (rare)
Pyruvate Kinase deficiency
•Affects RBCs (chronic hemolytic anemia) b/c RBC is dependent on glycolysis
•Believed to affect Na+/K+ ATPase pump and other ATP-dependent processes. Not enough ATP to move ions
–What effect will prolonged high-intensity exercise have on his muscles?
increase ATP and increase mitochondria
increase rate of waste removal and increase rate of lactic acid removal
Predict the effect of elevated intracellular levels of each of the following on ATP production by glycolysis:
AMP: stimulate pkf
acetyl coa: decrease pdh
nadh: decrease pdh
glucose 6 phosphate: decrease hexokinase
fructose-1,6- bisphospahte: incerase pyruvate kinase
Toward the end of running the 1600m during a track meet, a 22-year-old athlete cramps up. In biochemical terms, what is causing the cramping and what will alleviate it?
increase lactic acid associates to lactate and H+ so pH decreases.
we need to slow down to breath to increase O2
Glycolysis and the TCA cycle make some ATP, but their BIGGEST contributions to ATP production are NADH and FADH2......why
Because delivery of electrons to the ETC is needed to drive oxidative phosphorylation.
______ from glycolysis in the cytoplasm enters the mitochondrial TCA cycle as ____
Pyruvate from glycolysis in the cytoplasm enters the mitochondrial TCA cycle as acetyl-coA
The ______ complex is the enzymatic between glycolysis and the TCA cycle – produces 2 NADH
•The pyruvate dehydrogenase complex is the enzymatic linker between glycolysis and the TCA cycle – produces 2 NADH
The TCA cycle operates under
aerobic conditions
TCA Cycle Products
2 GTP
6 NADH
2 FADH2
ΔG from ATP hydrolysis can be used to do work
–Mechanical, motility
–Transport
–Synthesis
–Biochemical
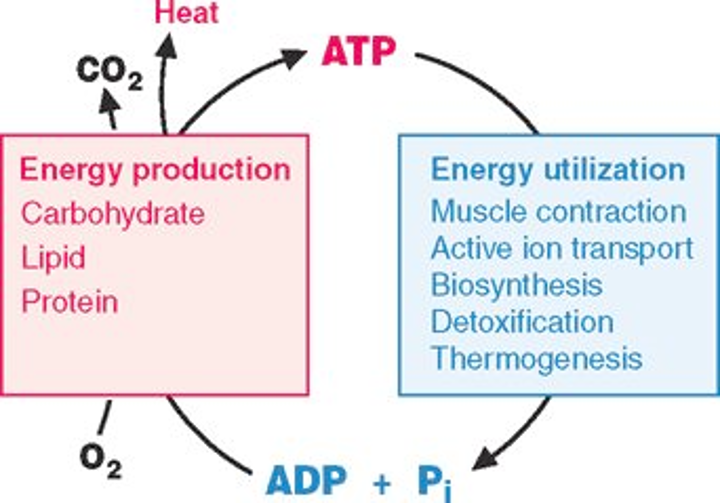
body gets energy by breaking down carbohydrates, lipids (fats), and proteins.
O₂ (oxygen) is consumed.
CO₂ (carbon dioxide) and heat are released as byproducts.
Energy is captured in the form of ATP
ATP is then used to power critical cellular processes:
Muscle contraction – movement, posture, heart beating.
Active ion transport – pumping ions across membranes (e.g., Na⁺/K⁺ ATPase).
Biosynthesis – making proteins, nucleic acids, lipids.
Detoxification – breaking down harmful substances.
Thermogenesis – generating heat to maintain body temperature.
When ATP is used, it is converted back into ADP (adenosine diphosphate) + Pi (inorganic phosphate)
What drives ATP muscle contraction?
hydrolysis
T or F - Cell will never be zero at ATP
true
How does PDH deficiency differ from PK deficiency? What lab results would you expect for PDH vs PK?
PK: glycolytic enzyme, 1st cell type attacked = RBC
PDH: linker step after glycolysis before TCA. Problems: lactic acidosis, increase pyruvate alanine, hypotonia, leathery, neurological defects
Dinitrophenol (uncoupler)
Dissipate the proton gradient by allowing H⁺ to flow back into the matrix without driving ATP synthase.
Result: ETC speeds up (↑ O₂ consumption, but ATP is not produced.
Energy lost as heat.
Dinitrophenol (DNP): an uncoupler; facilitates proton transfer across the inner mitochondrial membrane. Used for weight loss
Clinical effects: uncoupling oxphos → heat production, hyperthermia, possible damage to organs. Respiration will increase
ETC inhibitors
Electron Transport Chain (ETC) InhibitorsMechanism:
Block electron flow through a specific complex in the ETC.
Result: ↓ proton gradient, ↓ ATP synthesis, and no change in oxygen (not used) so cannot form water
ETC inhibitor examples, rotenone amytal, carbon monoxide, cyanide