Chemistry IGCSE - Acid, bases and salts
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Acid reacting with alkaline
Acid + alkaline → water + salt
Acid reacting with base
Base + acid → water + salt
Acid reacting with carbonates
Carbonates + acid → water + salt + carbon dioxide
Acid reacting with metals
Metals + acid → salt + hydrogen
Acid reaction mnemonics

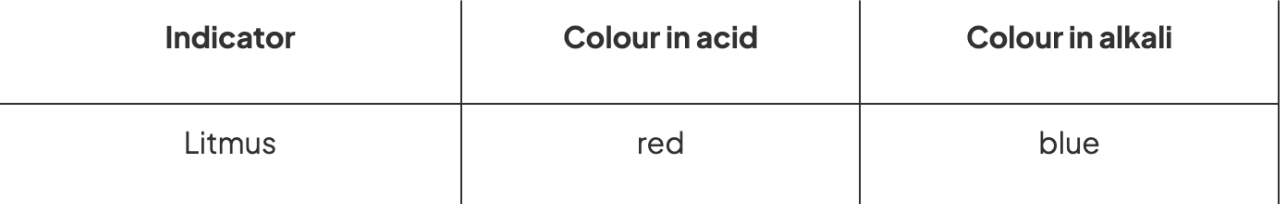
Indicators in litmus paper
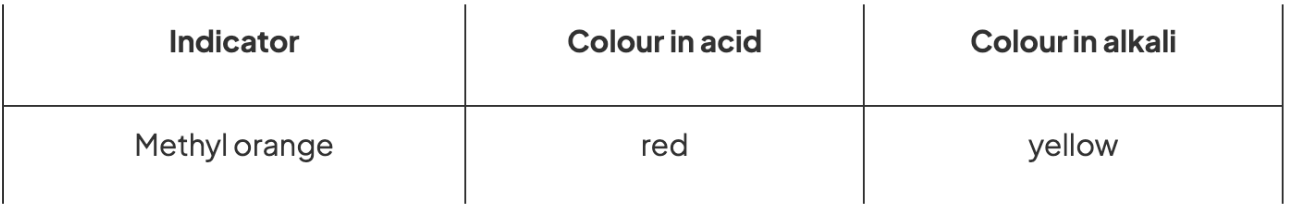
Indicators in methyl orange
Bases are oxides or hydroxides of metals
Alkalis are soluble bases
Neutral solutions’ pHs
pH: 7.0
Colour: green
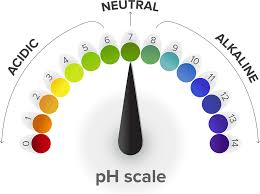
Acid solutions’ pH
pH < 7 → lower the pH → stronger the acid
Colour: changes from green → yellow → orange → red → acidity increases
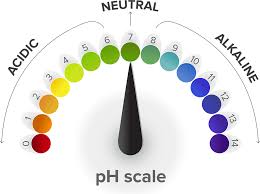
Bases solutions’ pH
pH > 7 → higher the pH → stronger the alkali
Colour: changes from green → blue → purple → alkalinity increases
Acid
has H⁺ ions
Bases
has OH⁻ ions
Neutralisation
acid + alkali → salt + water
Metal oxide → basic oxide
basic oxides → e.g. CuO + CaO
Basic oxides → acidic oxides
acidic oxides → SO2 + CO2
Amphoteric oxides
oxides that react with acids + with bases → produces salt + water
→ e.g. Al2O3 + ZnO
Describe the preparation, separation + purification of soluble salts by reaction of an acid with: an alkali by titration
Pipette a measured volume of alkali into the flask, add indicator
Titrate with acid until indicator just changes colour → record volume used
Repeat → mix fresh alkali + exactly that volume of acid without indicator
Gently heat to concentrate → cool to crystallize
Filter off crystals, wash + dry
Describe the preparation, separation + purification of soluble salts by reaction of an acid with: excess metal
Add small pieces of metal to excess acid until bubbling stops
Filter to remove unreacted metal → collect clear filtrate
Heat to concentrate → cool to form crystals
Filter, wash + dry
Describe the preparation, separation + purification of soluble salts by reaction of an acid with: excess insoluble base
Stir excess solid base into the acid until no more dissolves
Filter off undissolved base → keep filtrate
Concentrate filtrate by gentle heating → cool to crystallize
Filter, wash + dry
Describe the preparation, separation + purification of soluble salts by reaction of an acid with: excess insoluble carbonate
Add excess carbonate to acid until CO₂ evolution ceases
Filter off leftover carbonate → clear filtrate remains
Gently heat to concentrate → allow to cool & crystallize
Filter crystals, wash + dry
Hydrated substance
a substance that is chemically combined with water
Anhydrous substance
a substance containing no water
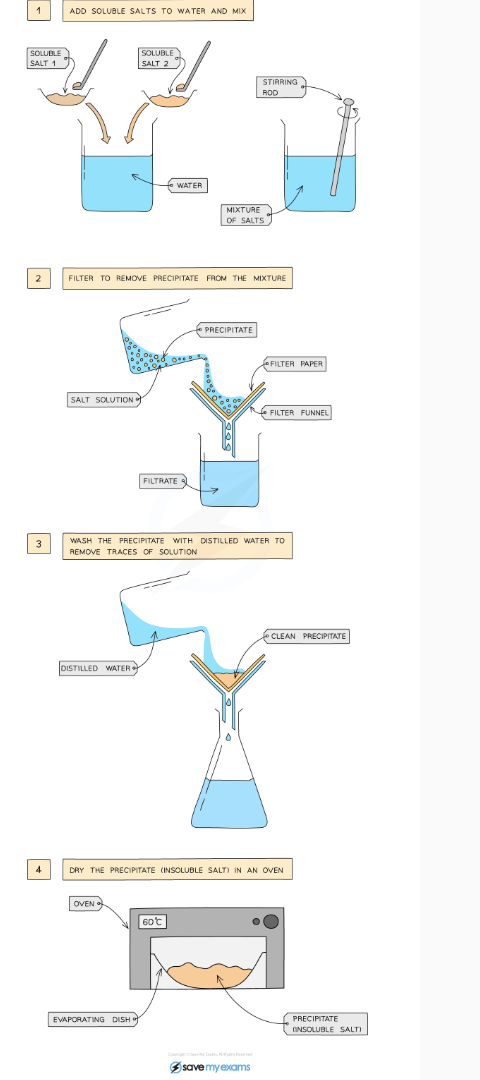
Preparation of insoluble salts by precipitation
Dissolve soluble salts in water → mix together using a stirring rod in a beaker
Filter to remove precipitate from mixture
Wash the residue with distilled water → remove traces of other solutions
Leave in an oven to dry