Chemistry Unit 4
1/83
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
84 Terms
What is the concentration of a solution?
Equal to the mass of solute divided by the volume of the solvent
Units are grams/100 mL
What is the solubility of a solution equal to?
The max concen. of the solute-how much it can take before precipitate is formed
What do you think the solubility curve would look like for sugar? Explain.
I think that the solubility curve for sugar would look much like that of potassium nitrate, where it increases exponentially with the temperature increase. I think this because the intermolecular forces in sugar are weak (compared to something like NaCl), therefore allowing the solubility to increase more with temperature.
How will the solubility of a compound change?
Solubility of a compound will change as the temp changes
What can help us exactly what the solubility of a compound is at a given temp?
A solubility graph
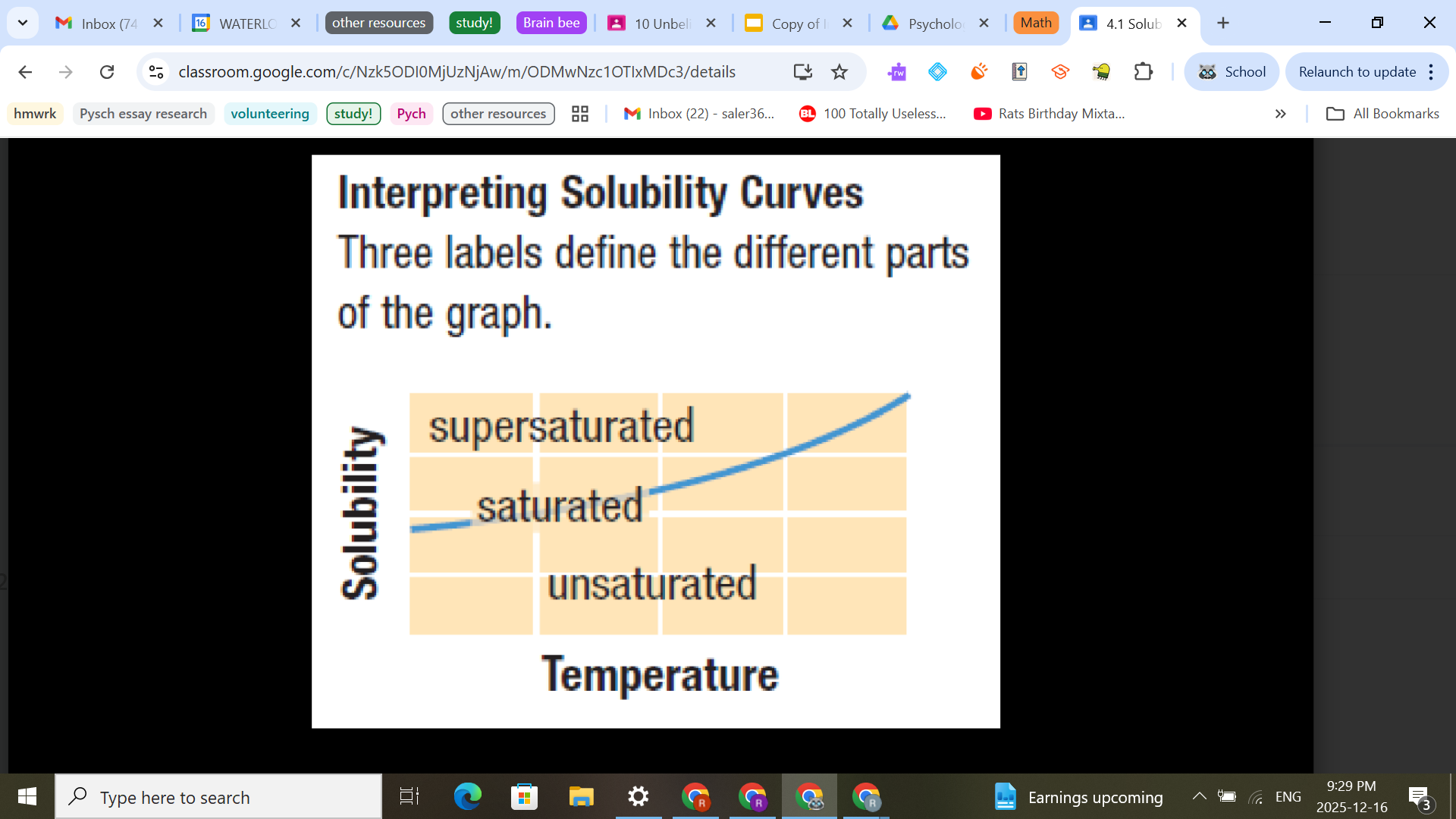
How to read a solubility graph?
Points directly on the curve represent a saturated solution.
Points below the curve represent an unsaturated
solution.
The vertical difference (subtraction) between a point below the curve and a point on the curve represents the additional mass of solute/100 g H2O required to saturate the solution at that temperature.
Points above the curve represent a supersaturated solution.
The vertical difference (subtraction) between a point above the curve and a point on the curve represents the mass of solute/100 g H2O that will crystallize out of the solution at that temperature (basically says when precipitate will be formed)
Solubility in water-include info abt solid, gas and diff types of liquid solubility in water and its relation to temps
Solids have a higher solubility in water at higher temperatures (ionic and molecular)
Gases always have higher solubility in water at lower temperatures (inverse and linear relationship)
Polar liquids dissolve in water, solubility usually increases with increasing temperature
Non-polar liquids, do not dissolve, they are immiscible (form a separate layer)
Liquids containing small polar molecules (with H bonding) dissolve in water, they are miscible (mix well in all proportions)
Elements have low solubility in water e.g. Carbon is used in water filtration systems
Halogens and oxygen dissolve to a very tiny extent but they are still very reactive even in small concentrations.
How to find percent of solute in a solution?
percent of solute = (mass of solute/mass of solute + mass of solvent) x 100%
How to find mass of smth able to dissolve in a diff amount of water than ur given-eg. need 200g of water but u have 100g in the graph
use ratios-eg.: x/200g = 30/100g=60
30 would be the amnt able to be dissolved in 100g and ya
anyway same thing goes if ur finding water too so if ur asked smth like finding how many g of water u need to dissolve lets say 46.6g of a substance u’d do;
x/46.6 = 100/80
80 in this case being how many g of ur substance can dissolve in 100 g of water at ur given temp and then water is the x
Solution
Homogeneous mixture-uniform comp./same throughout
Can also be described as a mixture of substances appearing as one phase
Variable compositions with diff ratios of solvent to solute
Solutions can be dilute (small amounts of solute (being dissolved)) or concentrated (large amounts of solute)
Solvent
Any substance that has other substances dissolved in it
Usually the substance that’s present in the largest amnt. in a solution
Aqueous solution
A solution in which water is the solvent (dont the dissolving)
Miscible
Describes liquids that dissolve readily in each other in any proportion
Eg. water + ethanol-any amnt. of water will dissolve in any amnt. of ethanol- Described as “miscible” with each other
Immiscible
Liquids that do not readily dissolve in each other
Eg. Oil and water
Alloys
Solid solutions of metals
Amalgam
An alloy that is made of a metal dissolved in mercury
Solubility
The amnt. of solute that dissolves in a given quantity of solvent at a certain temperature
Usually expressed in g/100mL
Eg. The solubility of NaCl at 20 degrees C is 36g/100mL of water
Saturated solution
Formed when no more solute will dissolve in a solution-if u keep adding solute after this point precipitate will start to form (if it’s a solid dissolved in a liquid) due to the ecess solute
Unsaturated solution
A solution that is not yet saturated-can still dissolve more solute
Distillation
A method of separating solutions using differences in BPs (boiling points)
Will filtration separate solutions?
No
What gas is found as the greatest proportion in air?
Nitrogen
Solution of gases
Air
What is water also known as?
The universal solvent
Exothermic
Reaction that releases energy-has excess energy
Endothermic
Reaction that requires energy to continue
Chloroform
A poisonous non-polar solvent (doing the dissolving)
What does stirring affect?
The rate of solubility
Solute
Substance present in the smallest amnt-being dissolved in a solution
Can have multiple solutes in one solution
3 examples of solutions (solute in solvent)
Gas in Gas-eg. Oxygen in Nitrogen
Gas in liquid-eg. CO2 in pop (fizzy drink)-the gas is what makes it fizzy
Solid in Solid-eg. Zinc in copper-makes brass
Supersaturated solution
A solution that contains more dissolved solute than a saturated solution at the same temp.
This usually occurs when a temp. change occurs
These solutions are unstable and a disturbance can precipitate out the excess solute
What are the 3 degrees of solubility?
Soluble: more than 1g is dissolved in 100mL (>1g/100mL)
Slightly soluble: between 0.1g to 1g is dissolved in 100mL
Insoluble: Less than 0.1g is dissolved in 100mL of solvent (<0.1g/100mL)
What can solubility be explained in terms of?
Can be explained in terms of the forces that act between the particles of the substance in solutions
The formation of most solutions depends on the relative strength of three categories of forces:
Forces that attract particles of the solute to each other
Forces that attract particles of the solvent to each other
Forces that attract particles of the solute to particles of the solvent
6 factors affecting solubility
Ion charge
Ion size
Molecular size
Temperature
Pressure
Type of solvent
TIIM TP
Ion charge-in factors that affect solubility
Small charges tend to be soluvle, while ions with large charges tend to be insoluble
Eg. Na+ vs Fe 3+- an ion with a multiple charge increases the nuclear forces-attraction of the nucleus to the outer electrons- holding the ions together in a compound, resulting in Fe 3+ being less soluble than Na+-cause attraction within the atom is higher electrons leave less easily making it harder to be dissolved
Ion size- in terms of factors affecting solubility
As ion size increases, solubility increases too
Compounds with small ions tend to be less soluble
Large ions have weak bonds-easier to dissociate
Note: the size of an ion increases as u go down a family
Molecular size- in terms of factors affecting solubility
Small molecules are usually more soluble than large ones in polar solvents like water- cause if u think of it like a tape, a small tape can be unwrapped faster than a bigger tape cause its just less work- therefore, it dissolves
This is cause solubility depends on how well the solvent can pull particles apart and keep them separated
Solvent molecules (like water) have to surround each solute particle-therefore, a smaller molecular size=less surface area to surround and break apart which makes them easier to dissolve
Temperature- in terms of factors affecting solubility
Solids and liquids:
Solubility of solids increases with an increase in temp.-this is cause at higher temps, solvent particles have more energy. Therefore, more frequent energetic collisions occur with the solute particles-makes them break apart fast and easier-therefore more solubility
Gases in liquids:
Solubility of gases decreases with an increase in temperature- this is cause higher temps cause a large change in kinetic energy (movement=kinetic) of gas molecules when they enter/leave a solution-Higher temp of solution=higher the kinetic energy of the molecules
This allows the gas particles to escape (cause they’re moving more)
Anyway this is also what causes smth like a fizzy drink to lose its fizz when left out of the fridge or at warmer temps (this is cause it loses the dissolved CO2 faster when higher temps)
Pressure-in terms of factors affecting solubility
Only affects solubility of gases in liquids
When pressure increases, the solubility of gases increases
The solubility of a gas in a liquid is directly proportioned to the pressure of that same particular gas above the liquid-higher up=less pressure (think mountains-pressure is lower)
This is also why u get lightheaded when ur going up a mountain-cause the solubility of O2 in ur blood decreases (cause pressure drops)-this causes u to get less oxygen-making u lightheaded
Why does solubility of gases in liquids increase as pressure increases?
Gas molecules above a liquid are constantly entering and leaving the liquid-when pressure increases, these gas molecules get pushed closer to the surface of the liquid
This causes the the number of gas particles colliding with and entering the liquid to increase
This causes more gas to dissolve than to escape-increasing solubility
Eg. Carbonated drinks are bottled under high pressure so CO₂ stays dissolved
When you open the bottle, pressure drops → gas escapes → bubbles form
Type of solvent-in terms of factors affecting solubility
The general rule is “like dissolve like”
This means that a polar solvent will dissolve a polar solute or an ionic compound, while a non-polar solvent will dissolve a non-polar solute
Factors affecting the rate of dissolving
Temperature
- Increasing the temp. of the solvent increases the kinetic energy of the molecules which increases the number of collision between the solvent and the solute, and therefore the rate of dissolving
- Stirring brings fresh solvent into contact with solute which increases the number of collisions that solvent molecules have with solute molecules-increases dissolving
Particle size
-
What is concentration defined by? What are square brackets?
Concen. is defined as the amount of solute per quantity of solvent
Square brackets- [ ], means concentration of
Eg. [HCl] is the concentration of HCl
How to calculate concentration?
Calculation as Mass/Volume (m/v) percent
Calculation as Mass/Mass (m/m) percent
Concentration as Volume/Volume (v/v) percent
Parts per Million (ppm) and Parts per Billion (ppb)
Concentration calculation as Mass/Volume (m/v) percent
Gives the mass of solute dissolved in a volume of solution, expressed as a percent
Mass/Volume % = (mass of solute (g)/volume of solution (mL)) x 100%
Solid dissolved in a liquid (or gas dissolved in liquid)
Eg. M/V %= [ ] g/mol
Concentration calculation as Mass/Mass (m/m) percent
Gives the mass of solute divided by the mass of solution, expressed as a percent
Mass/Mass% = (m solute (g)/m solution (g)) x 100%
Solid dissolves in a solid
Concentration calculation as Volume/Volume (v/v) Percent
Gives the volume of solute divided by the volume of solution, expressed as a percent
Volume/Volume % = (V solute (mL)/V solution (mL)) x 100%
Liquid dissolved in liquid
Concentration calculation as parts per million (ppm) and parts per billion (ppb)
Describes the concentration of very small quantities
Usually expressed in terms of mass/mass relationships
ppm= (Mass of solute (g)/Mass of solution (g)) x 10^6 (cause 6 zeroes cause million get it)
ppb= (Mass of solute (g)/Mass of solution (g)) x 10^9-cause billion 9 zeroes get it
Note: Your final answer does not refer to the number of particles per million or billion, but rather the mass of solute compared to the mass of solution
Molar Concentration
Molarity (M) is the number of moles of solute dissolved per liter of solvent
The equation we use to calculate molar concentration is (as an eq triangle)
n
M V
M=Molarity (mol/L)
n=Number of moles (mol)
V=Volume of solvent (L)
Eg. [1.0], or 1.0M, this would be 1.0 mol of solute is dissolved in 1.0L of solvent (1/1)
Preparing Solutions and Dilutions
A standard solution is a solution with a known concentration ([ ])
There are 2 ways to prepare a solution:
1. Dissolve a measured mass of solute in a certain volume of solvent
2. Dilute a solution of known concentration
What is a useful tool in preparing solutions?
Volumetric flask-measures volume
It’s a pear-shaped glass with a flat bottom and a long neck
Volumetric flasks provide are very accurate tools for measuring volumes
How to prepare a solution
Determining the mass (solute) required to make the desired concen. ([ ]) and volume of solution
Measure out and dissolve the solute in approximately half the volume of solvent-doing the dissolving (usually water for us)-eg. if it was a 200mL flask, add 100mL of water
Raise the volume of your solvent to the desired total volume by adding more water
Example of solution prep-preparing a stock solution
How would you go about preparing a 0.250 L solution of 1.0 M of NaCl
n= MxV
= 1.00 mol/L x 0.250 L
= 0.250 mol
Mm= 22.99 g/mol + 35.45 g/mol
= 58.44 g/mol
find m:
m= nxMm
= 0.250 mol x 58.44 g/mol
= 14.61 g
How can u dilute a solution to make it less concentrated
Add more solvent (water)
Add less of NaCl (solute) to the same volume of solvent (water)
Dilution Calculations
Find the amnt of solution u need (total)-solute+ solvent
Find the solute u need
Top up with solvent
Involves preparing a diluted solution from a more concentrated stock solution
M1= Initial concen.
V1= Initial volume
M2= final concen.
V2= Final volume
Note: M1 and V1 are usually more concentrated-cause ur diluting it so final should be less
Dilution steps
Get a clean 2.00 L volumetric flask
Add half of the final volume-add 1.00L of water to the flask (so that with the solute it becomes 2.00L)
0.400 L of 10.0 M HCl, add a stopper to the flask and mix-shake around
Add H2O to the meniscus (like the line on the flask)
Note: go only to the BOTTOM of the curve
Add the stopper and mix-to get exactly 2.00L
Reactions in an Aqueous solution-include what an ionic compound will do when put in water (2 possibilities) and what that makes it, what happens when an ionic compound dissolves in water and what this is called, what a double displacement reaction is, where they occur, what they are caused by, how can we identify if a precipitate is formed, and hw we can show the process of a DD reaction
When an ionic compound is placed in water, most will dissolve, which means they are soluble in water.
Some ionic compounds will remain as a precipitate and are insoluble.
If an ionic compound dissolves in water, it means that the compound is temporarily splitting apart into its ions- This process is referred to as an ionic compound dissociation.
A chemical reaction between two aqueous solutions that contains ions is called a double displacement reaction.
Double displacement reactions occur in water, and are a direct result of ionic compounds dissociating into their ions- recall that a double displacement reaction will only occur if gas, water or a precipitate forms- If no reaction occurs, the ions stay in solution (as aq)
A precipitate is seen as sudden cloudiness or an obvious solid formed when 2 solutions are mixed- A solubility table can be used to predict which of the possible products formed is the precipitate.
We can show the step-by-step process of a double displacement reaction by writing out an ionic equation from the molecular equation.
There are several different components to an ionic equation.
Total Ionic Eq
Describes the # and type of ions in solution in the chemical reaction of the soluble compounds.
Net Ionic Eq
An ionic equation with no spectator ions, showing only the ions that react and the insoluble product (s) or precipitates that is produced. This can also be the formation of water in Neutralization reactions.
Spectator ion
Non-reacting ions that appear on both sides of the equation
Rules for Writing Net Ionic Equations:
Write the complete chemical Eq for the reaction
Rewrite the soluble (aq) ionic compounds as ions-eg. NH4Cl (aq) would be written as NH4+ (aq) and Cl- (aq)
Leave insoluble (precipitate) ionic compounds as formula units-eg. ZnS (s) would stay as ZnS (s)
Leave molecular compounds as molecular formulas since these compounds produce relatively few ions in an aqueous solution-eg. CO2 (aq) stays CO2 (aq)
Write all acids as formula units (keep them normal not as individual ions) except for 6 strong acids listed below, since they ionize almost completely in water, write them as ions
Cancel out spectator ions. Keep only covalent compounds, the ions that react (the ones present in the precipitate product), and the precipitate that forms in the reaction
Note: Any gas that is involved in the reaction must appear in the net ionic eq
Check that both the charges and the atoms are balanced in the net ionic eq
The 6 strong acids:
HCl- H+ (aq) Cl- (aq)
HBr- H+ (aq) Br- (aq)
HI- H+ (aq), I- (aq)
H2SO4- 2H+ (aq) SO42- (aq)
HNO3- H+ (aq) NO3- (aq)
HClO4- H+ (aq) ClO4- (aq)
Acids
Sour taste
React with certain metals to produce hydrogen gas (eg. magnesium)
React with carbonates and bicarbonates to produce CO2 gas (eg. sodium carbonate)
Corrode metals
Electrolytes
Turns blue litmus paper red
Turns BTB yellow
Conducts electricity
Bases
Bitter taste
Feel slippery-many soaps contain bases
Turns red litmus paper blue
Turns phenolphthalein pink
Turns BTB blue
Conducts electricity
Arrhenius Theory of Acids and Bases
An acid is a substance that dissociates in water to produce one or more hydrogen ions, H+
A base is a substance that dissociates in water to form one or more hydroxide ions, OH−
Acids increase the concentration of H+ in aqueous solutions. Thus, an Arrhenius acid must contain hydrogen as the source of H+
Bases increase the concentration of OH− in aqueous solutions. An Arrhenius base must contain the hydroxyl group, OH, as the source of OH−
The ion responsible for the properties of acids is the H+ ion and the ion responsible for the properties of bases is the OH- ion
Basically, Arrhenius acids must contain H+ and Arrhenius bases must contain OH-
Problems with Arrhenius model
Assumes that water is there in the reaction but doesn’t write it-however, if u were to write it, the H wouldn’t just be H+ it’d be hydronium-which is actually how a proton is supposed to exist so to say that its H+ for acids is kinda wrong-cause H2O must react in some way with H or Cl cause tis polar
Ammonia
- Ammonia is one of several substances that produce basic solutions in water. However, it does not contain hydroxide ions, but it does produce these ions when it reacts with water.
- Ammonia also undergoes a neutralization reaction with acids.
- The Arrhenius theory cannot explain the basic properties of ammonia. Nor can it explain the fact that certain other substances, such as salts that contain carbonate ions, also have basic properties.
limited to acid-base reactions in a single solvent, water. Many acid-base reactions take place in other solvents.
Bronsted-Lowry Theory of Acids and Bases
An acid is a substance from which a proton (H+ ion) can be removed.
A base is a substance that can remove a proton (H+ ion) from an acid.
This theory overcame the problems related to the Arrhenius theory
Like an Arrhenius acid, a Brønsted-Lowry acid must contain H in its formula. This means that all Arrhenius acids are also Brønsted-Lowry acids.
However, any negative ion (not just OH−) can be a Brønsted-Lowry base.
Water is not the only solvent that can be used (unlike the Arrhenius acids)
According to the Brønsted-Lowry theory, there is only one requirement for an acid-base reaction- One substance must provide a proton, and another substance must receive the same proton. In other words, an acid-base reaction involves the transfer of a proton.
Also, any substance can behave as an acid, but only if another substance behaves as a base at the same time. Similarly, any substance can behave as a base, but only if another substance behaves as an acid at the same time.
Eg. the reaction between hydrochloric acid and water- In this reaction, hydrochloric acid is an acid because it provides a proton (H+) to the water. The water molecule receives the proton. Therefore, according to the Brønsted-Lowry theory, water is a base in this reaction. When the water receives the proton, it becomes a hydronium ion (H3O+). Notice the hydronium ion on the right side of the equation.
Every acid has a conjugate base, and every base has a conjugate acid.
The conjugate base of the acid-base pair has one less hydrogen than the acid. It also has one more negative charge than the acid. The conjugate acid of the acid-base pair has one more hydrogen than the base. It also has one less negative charge than the base.
Conjugate acid-base pair
Two molecules or ions that are related by the transfer of a proton
Conjugate=being linked together
Conjugate acid
The conjugate acid of a base is the particle that results when the base receives the proton from the acid.
Conjugate base
The conjugate base of an acid is the particle that remains when a proton is removed from the acid
Eg. In the reaction between hydrochloric acid and water, the hydronium ion is the conjugate acid of the base, water. The chloride ion is the conjugate base of the acid, hydrochloric acid.
Match each characteristic of theory to either the Arrhenius of Bronsted-Lowry theory:
Characteristic of Theory | Arrhenius | Bronsted-Lowry |
Any anion can be a base | ||
An acid dissociates in water to form H+ | ||
An acid is a substance from which a proton, H+, can be removed | ||
A base forms ions in solutions | ||
Acid-base character is not dependent on water as the solvent | ||
Does not use concept of conjugate acid-base pairs | ||
Theory was proposed in late 19th century | ||
Acid behaviour and basic behaviour occur simultaneously |
Characteristic of Theory | Arrhenius | Bronsted-Lowry |
Any anion can be a base | Yes | |
An acid dissociates in water to form H+ | yes | |
An acid is a substance from which a proton, H+, can be removed | Yes | |
A base forms ions in solutions | yes | |
Acid-base character is not dependent on water as the solvent | Yes | |
Does not use concept of conjugate acid-base pairs | yes | |
Theory was proposed in late 19th century | yes | |
Acid behaviour and basic behaviour occur simultaneously | Yes |
How to find concentration of ions (using stoichiometry)
Find mols of the known compound
Find how many ions of the element in the compound (eg. how many Cl- ions in KCL)-this gives u ur ratio
Use the ratio from 2 to find mols of your element
Find concen. of your element-formula triangles
How to find minimum volume of reactant to precipitate (stoichiometry)
Balanced chemical eq
Find mols of known compound
Find mols of the compound ur looking for (mol ratios from BCE)
Find volume of the compound question is asking for
fact check this
Limiting reactants in solution reactions (stoichiometry)
BCE-to find precipitate
Find mols of both compounds
Use BCE coefficients to find limiting
Finding the mass of a precipitate
BCE
Find mols of known compound
Use mol ratios to find mols of precipitate
Use mol ratios to find mass of precipitate (n x Mm)
fact check
Neutralization reactions
Occurs when OH- (base) and H+ (acid) are mixed to make H2O and a salt
The general word eq is:
Acid + Base —> Water + Salt
Acid-Base Titrations
Titration:
A technique that involves the careful measuring of the volume of one solution required to completely react with a known quantity of another
Acid-base titration:
Measuring the volume of a base (of known concen) allows us to determine the molarity (concen) of the unknown acid
The base is poured into a burette and the acid is poured into a pipette
From the pipette, the acid is poured into an Erlenmeyer flask
An indicator is used to indicate when the neutralization reaction is complete
Phenolphthalein is the most common indicator used for titrations- it will be clear when added to the acid ad tur pink abruptly at pH 8 (when the reaction is complete)
Neutralization occurs at the first signs f the solution staying faint pink
The goal is to knw when the amnt of titrant (known solution) is just enough to react with all the acid/base the sample contains-this would be the equivalence point (ideally, this point and the endpoint are the same)
Note: Phenolphthalein is a good indicator for titrate a strong aid with a strong base or a weak acid with a strong base-cause will turn pink more obviously ig
Define the following terms:
Titrant
Analyte
Equivalence point
Endpoint
Titrant
A solution of known concen
Analyte
A chemical whose concen is being measure/unknown
Equivalence point
The time when the number of moles of a titrant= the number of moles of the analyte
Endpoint
Point where the indicator being used changes colour/reaction is complete
Strengths of aids and bases
Acids
The strength of an acid depends on the degree in which it dissociates to produce hydronium (H3O+) ions
Bases
The strength of a base depends on the degree in which it dissociates to produce hydroxide (OH-) ions
Strong acids:
>99% dissociation
Weak acids:
<50% dissociation
Strong bases
>99% dissociation
Weak bases:
<50% dissociation
Note: strong acids are more dangerous than weak acids because the corrosive behaviour of acids is due to hydronium ions
How to calculate pH/strength of acids and bases
pH= -log[H3O+]
[H3O+] = 10-pH
if ur given POH, POH + pH= 14 (just subtract to find pH and use formulas)
Redox reaction
Many reactants involve the transfer of electrons form one species to another
Oxidation number rules (redox)
Ox # of hydrogen is +1
Ox # of group 1 elements is +1
Ox # of oxygen is -2
Ox number rules of atoms and molecules
Ox # of an ion is always the superscript (up top)
The ox # of an element ON ITS OWN is 0
The sum of all ox # in a compound/molecule is 0 (unless stated with a charge/state otherwise)
Define the following:
Oxidation, reduction, oxidizing agent, reducing agent
Oxidization
Loss of electrons
Reduction
Gain of electrons
Oxidizing agent
Gains electrons and is reduced in the chemical reaction
Reducing agent
Loses electrons and is oxidized in the chemical reaction
Why is a volumetric flask use in a titration rather than