paper 1: questions&ms
1/3
Earn XP
Description and Tags
jun 22
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
explain why the pH of an acid depends on (4):
the strength on the acid
the concentration of the acid
the stronger an acid the greater the ionisation/disassociation (in aqueous solution)
so stronger acids have a lower pH
the more concentrated an acid is the more acid/solute in the same volume of solution
so more concentrated acids have a lower pH
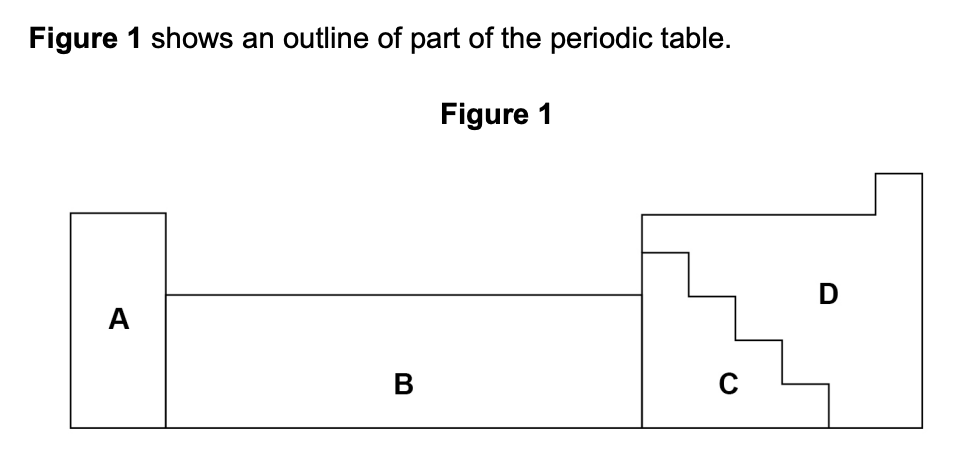
element R forms ions of formula R2+ and R3+.
which section of the periodic table in figure 1 is most likely to contain element R? (1)
B (transition metals)
[transition metals can form more than one ion]
give two differences between the physical properties of the elements in group 1 and those of the transition elements. (2)
group 1 elements:
have lower melting/boiling points
have lower densities
are less strong
are softer
q