Antibodies, molecular motors, + muscle contraction + protein analysis
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
what are antibodies produced by?
B-cells
____ is the most abundant antibody
IgG
each IgG consists of ____ subunits/ polypeptide chains
4
each IgG consists of ____heavy & light polypeptide chains
2
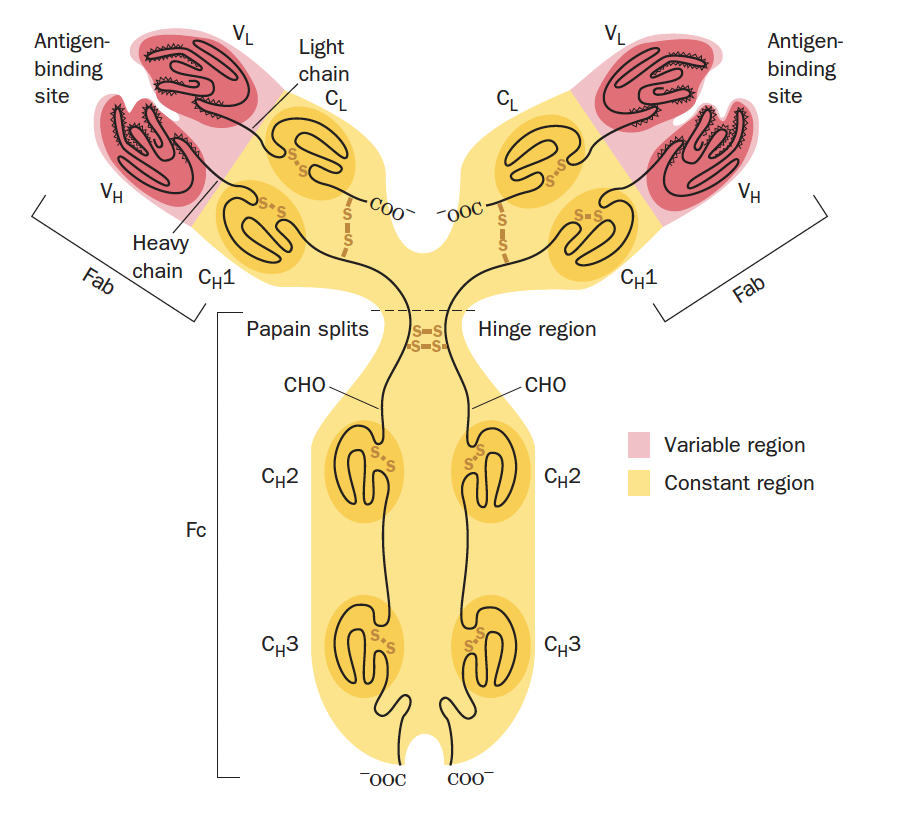
how are the chains in IgG kept together?
Within immunoglobulin folds, disulphide bonds form a Y shaped protein (cystines)
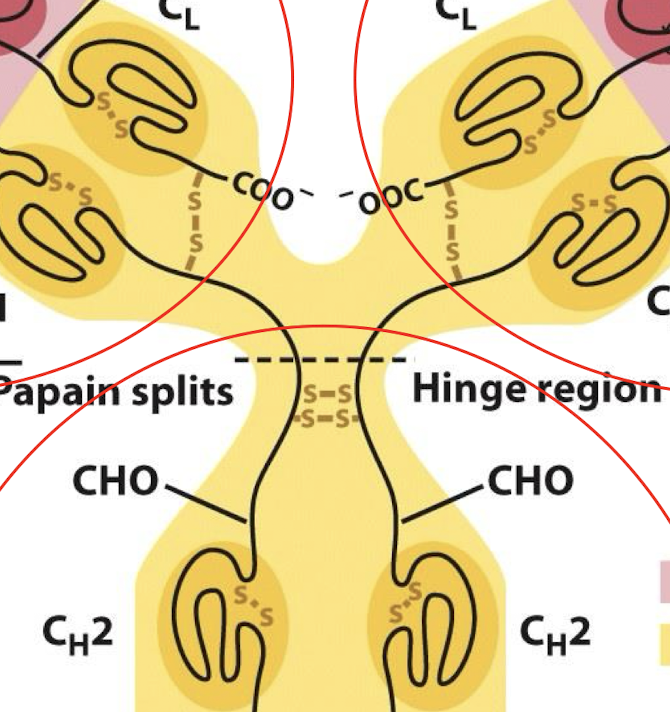
Disulphide bonds (cysteines) between chains are called _______
interchain
Disulphide bonds within chains are called _______
intrachain
CDR stands for _________________ each containing 3 _______________ (amino acid sequences highly variable) that bind the antigen.
complementarity-determining regions , hypervariable loops
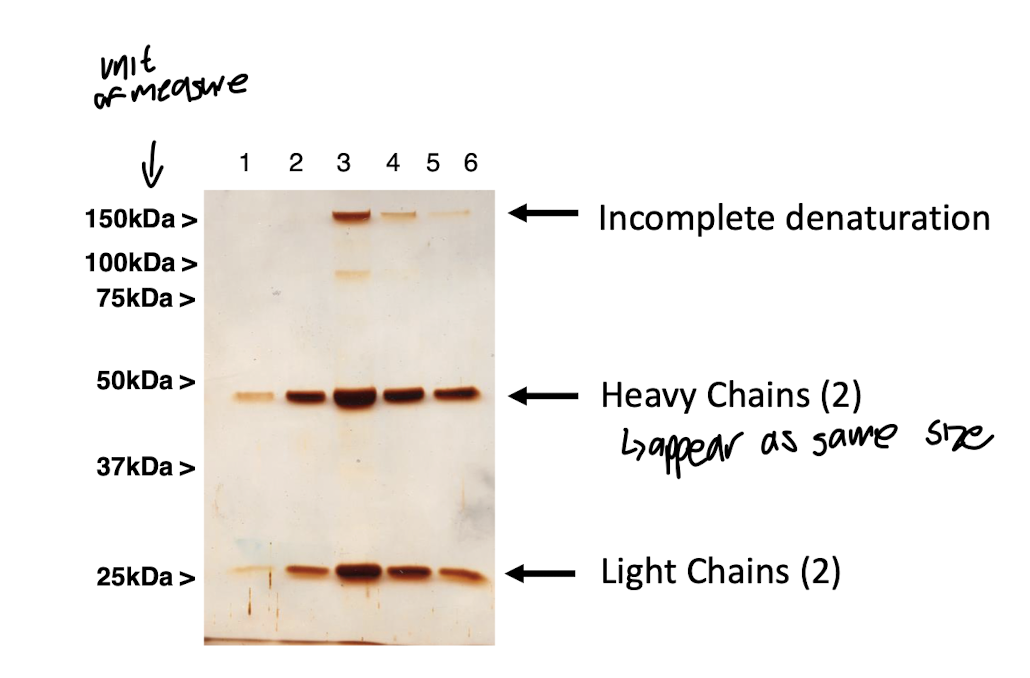
reducing agent used for PAGE (Polyacrylamide Gel Electrophoresis) of IgG
DTT : breaks disulphide bonds so subunits separate
the ______ on an antigen is capable of eliciting an ______ response and can combine with a specific antibody produced by such a response.
epitope, immune,

what is the term describing how IgG can bind to 2 antigens simultaneously?
divalent bonding
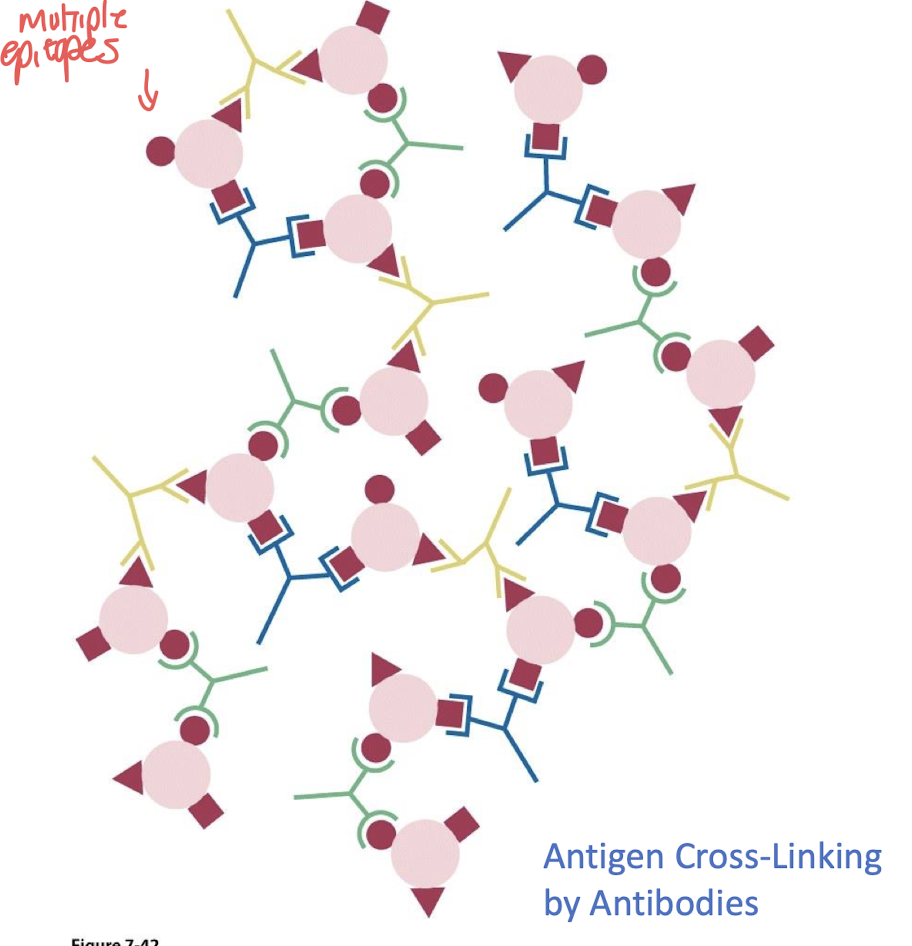
what are the 2 reasons antibodies cross link to form a lattice ?
- speeds up removal of antigen
- triggers B-cell proliferation
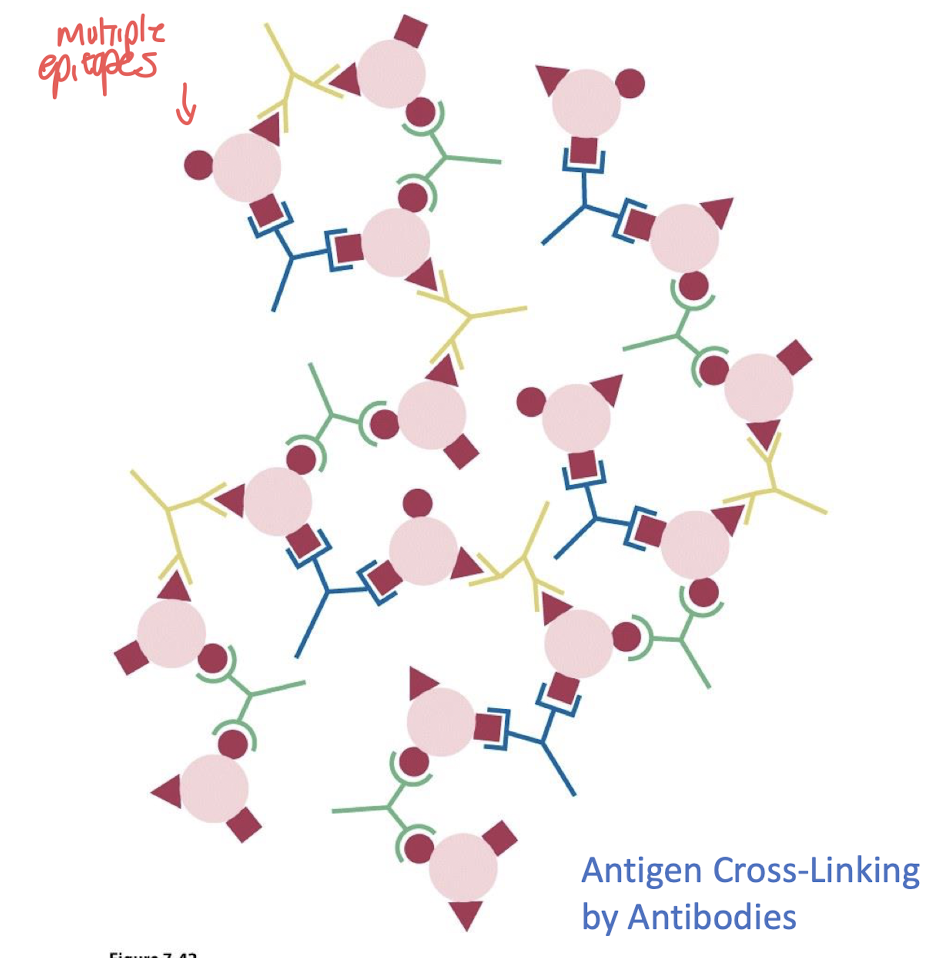
monoclonal antibodies are a collection of _______ that interact with a ____ antigen site
identical antibodies , single antigen site
what are 3 uses for lab produced monoclonal antibodies?
- protein purification and identification in the lab
identification of disease
therapeutic agents
Sickle cell anaemia is a genetic disorder caused by a specific ______ mutation in the gene that codes for _____
amino acid mutation, beta-globin
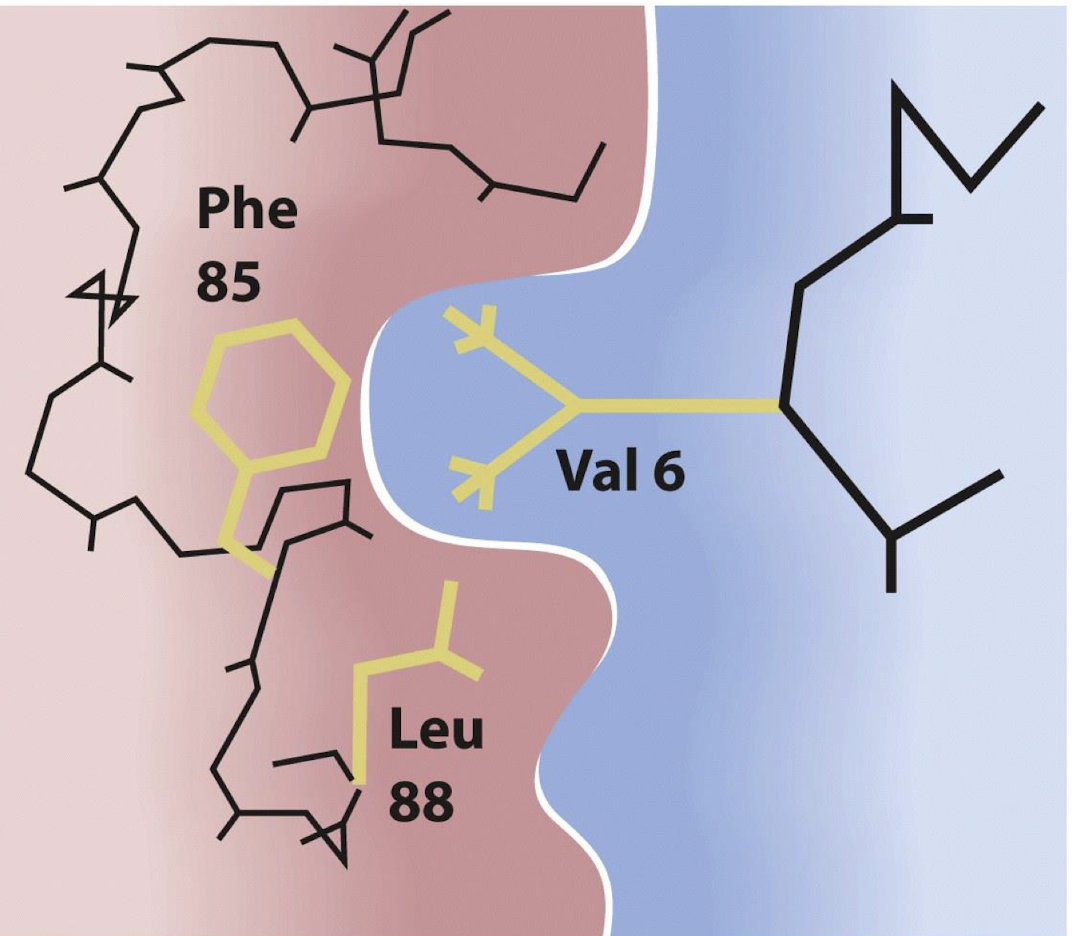
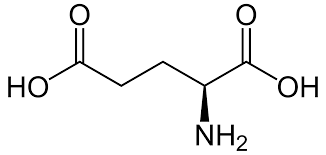
In normal haemoglobin, the 6th amino acid in the beta-globin chain is
glutamic acid (Glu)
in sickle cell anaemia, a single nucleotide change leads to the substitution of glutamic acid with ________
valine (Val)
the substitution of Glu with Val in sickle cell anaemia results in the formation of ________
abnormal haemoglobin called haemoglobin S (HbS).
Misfolded proteins have a tendency to ________
aggregate, meaning they stick together and form clumps.
insoluble clumps formed from misfolded proteins aggregating are called
amyloid (fibrils) eg β amyloid plaques (Alzeimher’s)
what are 3 ways amyloid plaques can disrupt cell function?
Interfere with cell-to-cell communication and signalling pathways.
Trigger inflammation and immune responses.
Induce oxidative stress, causing damage to cellular components.
The amino acid deletion that results in cystic fibrosis is caused by the loss of ____ at position ____ in the _____ protein
The loss of phenylalanine at position 508 in the CFTR protein (Cystic Fibrosis Transmembrane Conductance Regulator)
Muscle fibres are multinucleated, True or False
True
what are muscle fibres produced by?
cell fusion
________ are microscopic protein filaments that make up muscle cells
Myofibrils
Myofibrils are surrounded by flattened _____ containing Ca2+ called ________________ (nerve impulses cause Ca2+ release)
vesicles, sarcoplasmic reticula
Myofibrils consist of :
I band contains only thin filaments
A band contains only thick filaments in the H zone. Darker outer segments contain overlapping thick and thin filaments
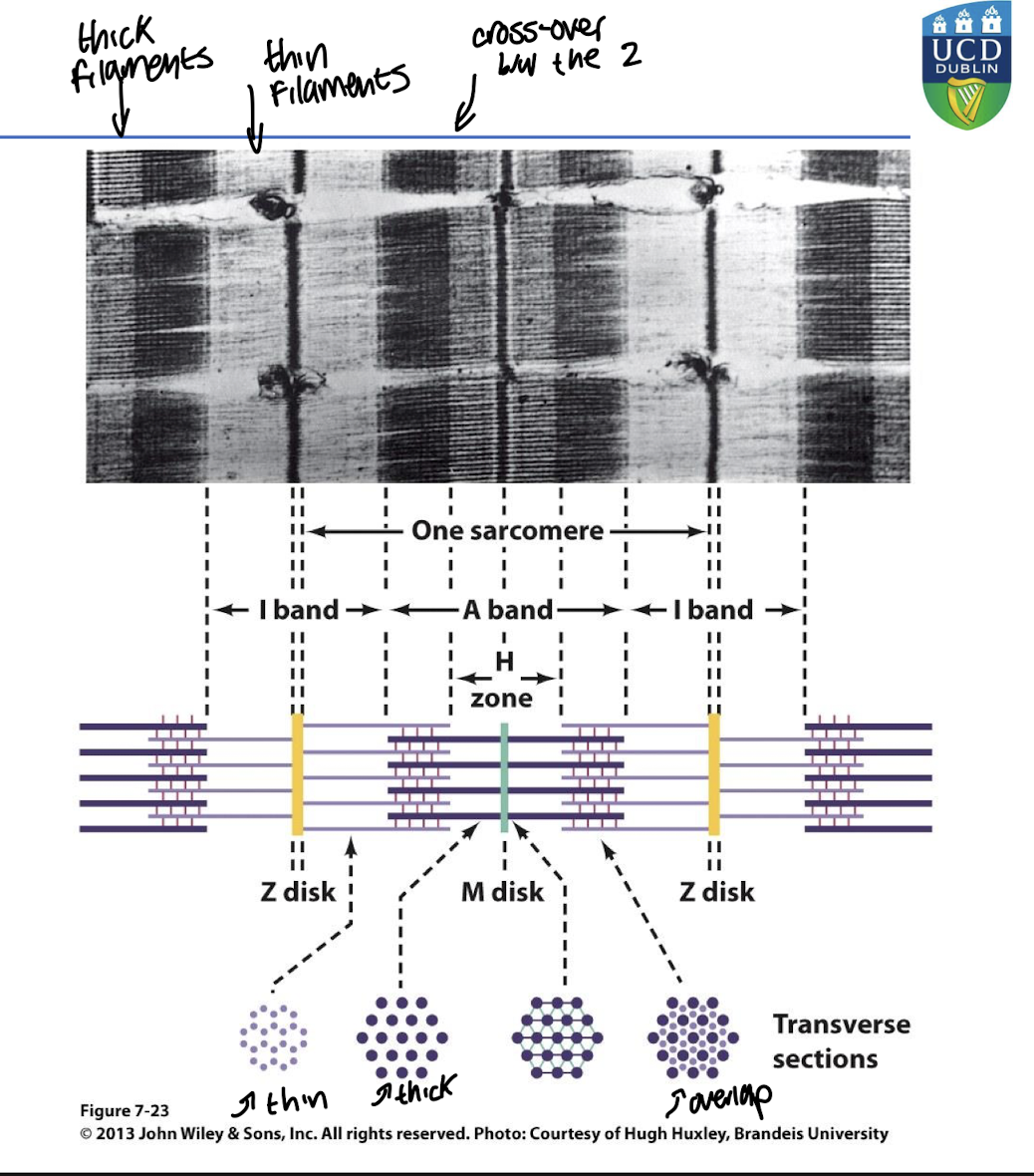
The _______ is the contractile unit of muscle
sarcomere
during muscle contraction, does the length of each filament of myofibril change?
Yes -
during muscle contraction, does the length of the sarcomere change?
Yes, it reduces in size due to overlapping/sliding of the filaments
what are the 4 key proteins involved in muscle contraction?
- myosin
- actin
- Tropomyosin
-Troponin
where is myosin found?
in the thick filaments
where is actin found?
thin filaments
where are Tropomyosin & troponin found?
thin filaments
what kind of role do Tropomyosin & troponin have?
inhibitory - they are regulatory proteins in muscle contraction.
The most abundant cytosolic protein in eukaryotes is _____ and its microfilaments are responsible for :
Actin
• Changes in cell shape
• Cell division
• Cellular locomotion
• Endocytosis
• Organelle transport
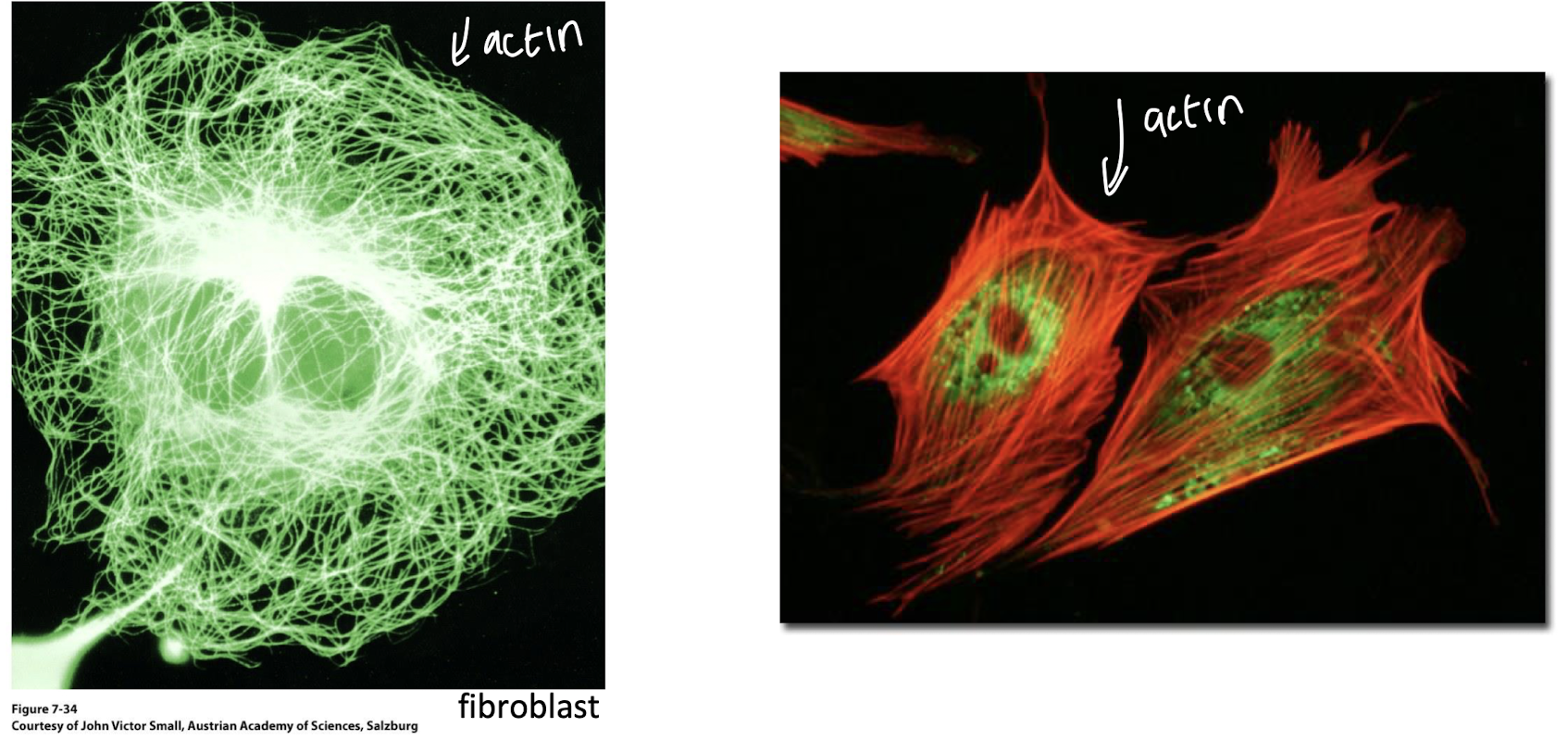
monomeric form of actin is known as ______ and forms polymers of _______ actin
G-actin, fibrous (F-actin) aka microfilaments
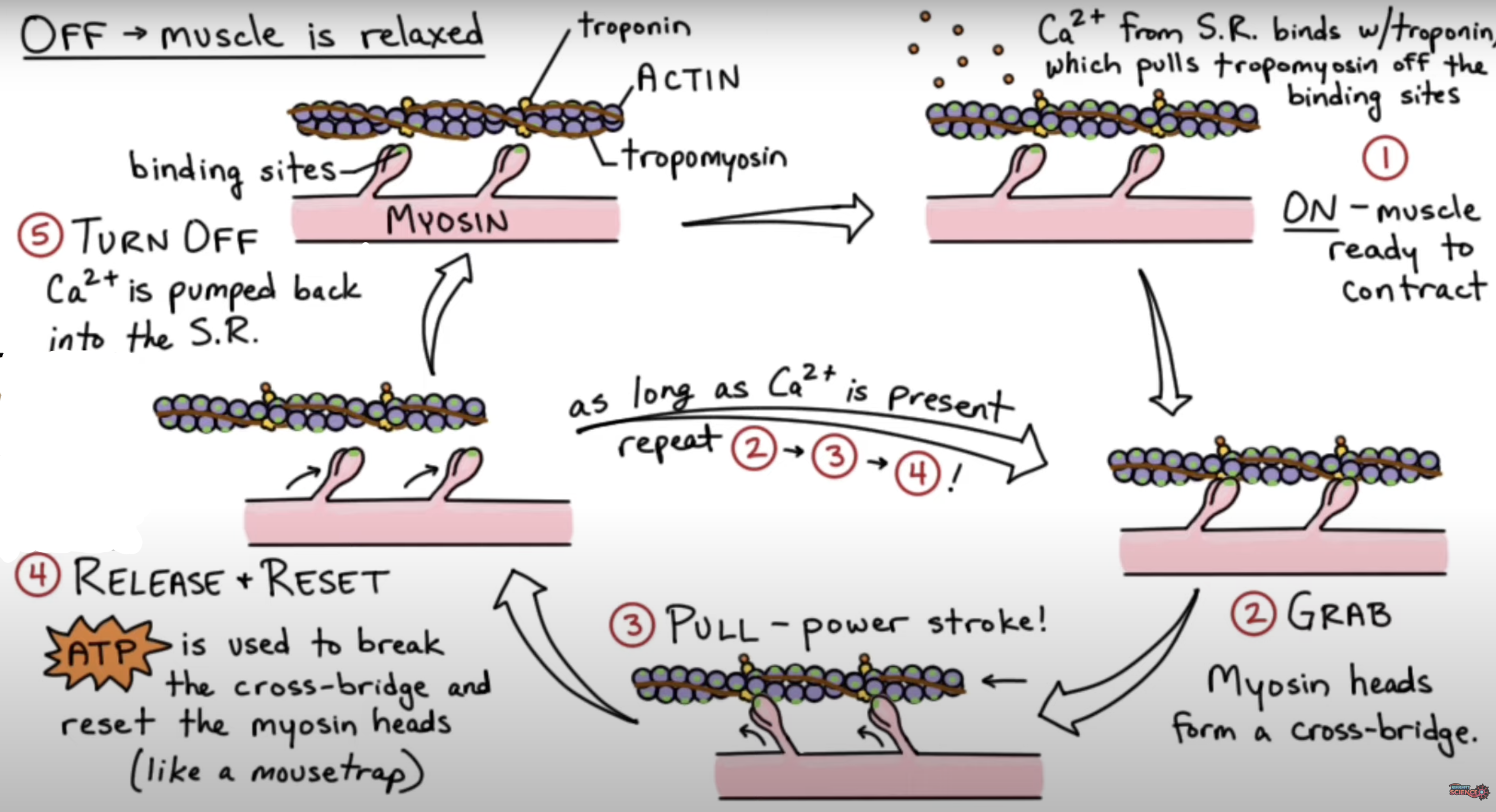
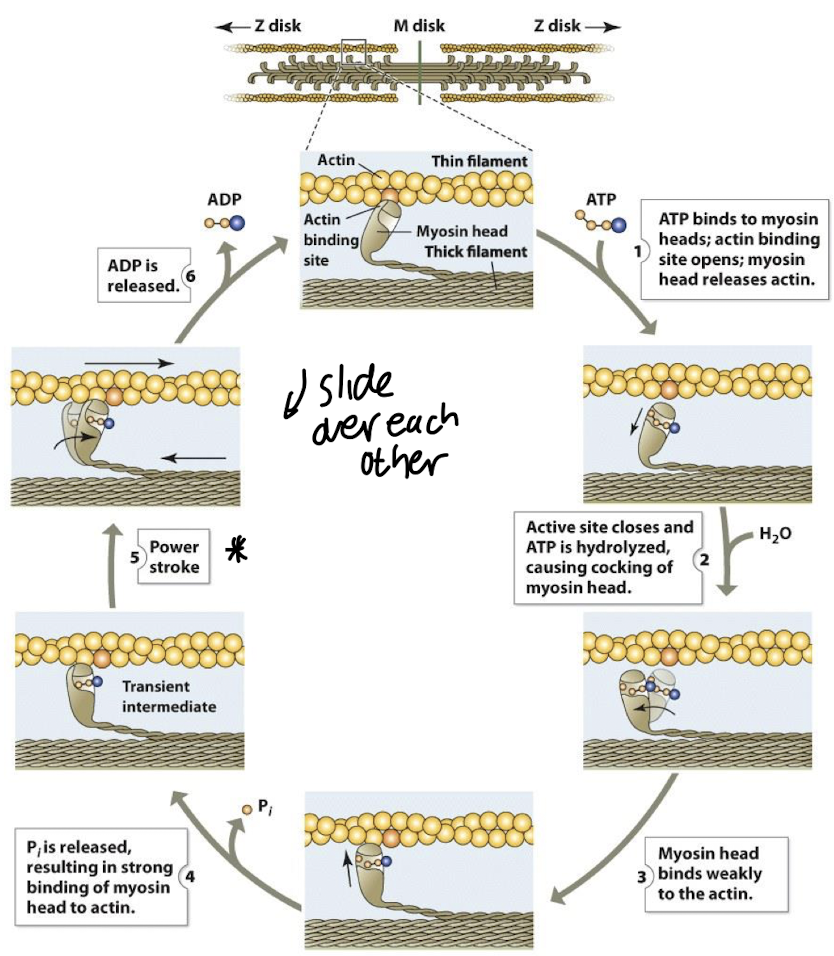
The sliding filament model of muscle contraction describes:
the movement of thick filaments (myosin) relative to thin filaments (actin)
• The myosin head must repeatedly detach and then reattach itself at a new binding site on actin further along the thin filament.
•Ca2+ ions from the Sarcoplasmic Reticulum bind to troponin, pulling tropomyosin off the binding sites.
• MH 1st bind weakly to actin forming a cross-bridge, when Pi is released, strong binding occurs & the MH pulls strongly onto actin creating a power stroke
• Contractile force is provided by ATP hydrolysis, which breaks the cross-bridge & resets the MH to their original position, ADP is released (chemical energy -> mechanical energy
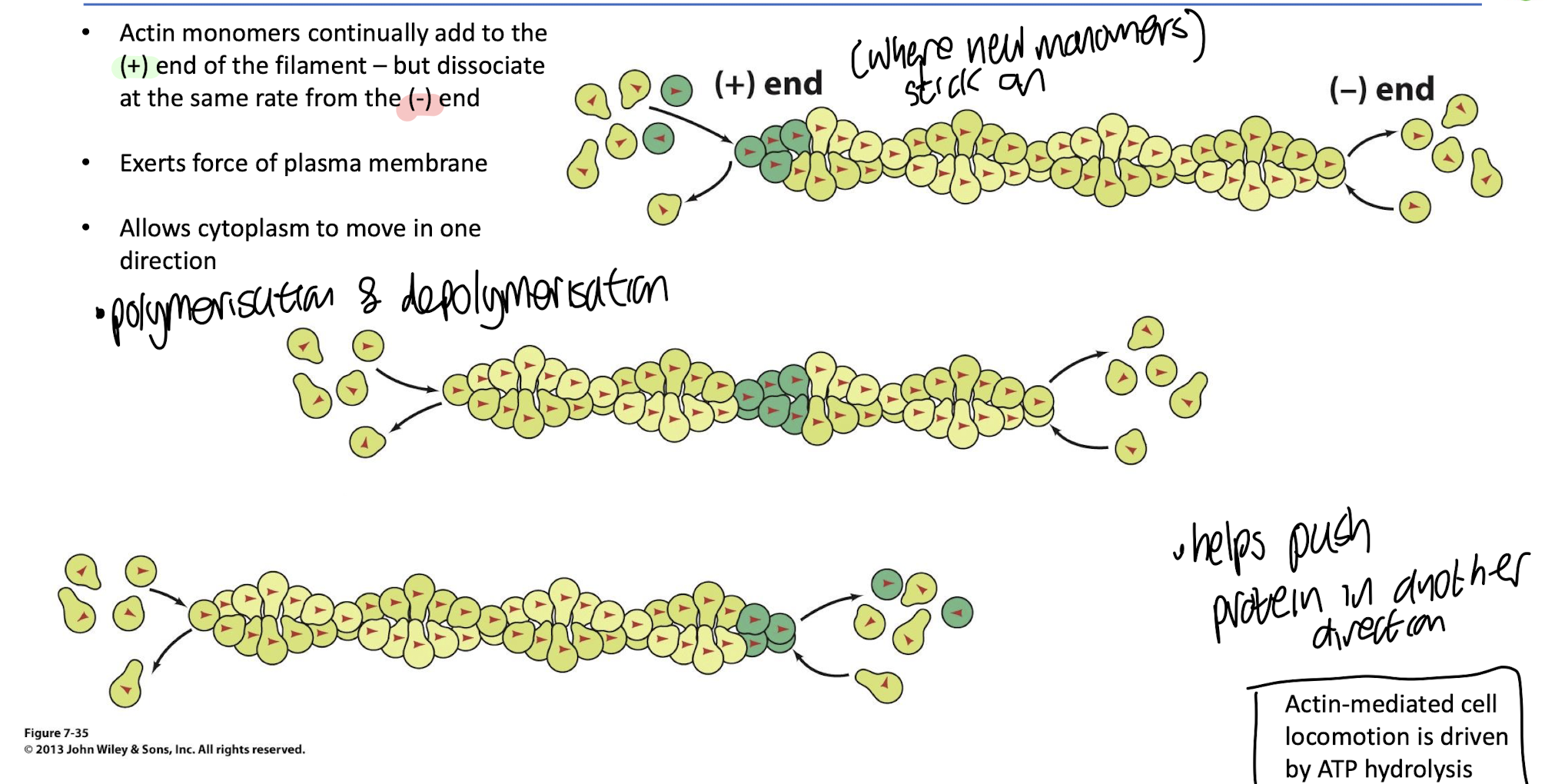
Cellular locomotion is mediated by:
treadmilling of actin microfilaments which is driven by ATP hydrolysis
How does Spectroscopy quantify proteins?
- aromatic side chains in amino acid residues absorb UV light
- these then emit light at a new wavelength
4 principle steps in ELISA
1) An antibody against the protein of interest is immobilised on the bottom of the wells.
2) The sample is added to the wells. The antibody binds the protein of interest, and other proteins are washed away.
3) A 2nd antibody (specific for a separate site on the protein of interest) is added to the wells. This antibody has an enzyme is attached. The unbound antibody-enzyme complex is washed away.
4) Binding of the 2nd antibody- enzyme complex is measured by assaying the activity of the enzyme.
*The amount of substrate converted to product indicates the amount of protein present.
proteins are separated in a lab by _____ in a __________ with a ________ gradient
spinning , centrifuge, in a salt gradient
3 steps in the fractionation of proteins:
A salt is added to a solution of macromolecules to a concentration just below the precipitation point of the protein of interest
After centrifugation, the unwanted precipitated proteins are discarded and more salt is added to the supernatant to a concentration sufficient to precipitate the proteins of interest
After a 2nd centrifugation, the desired protein (green) is recovered as a precipitate and the rest is discarded
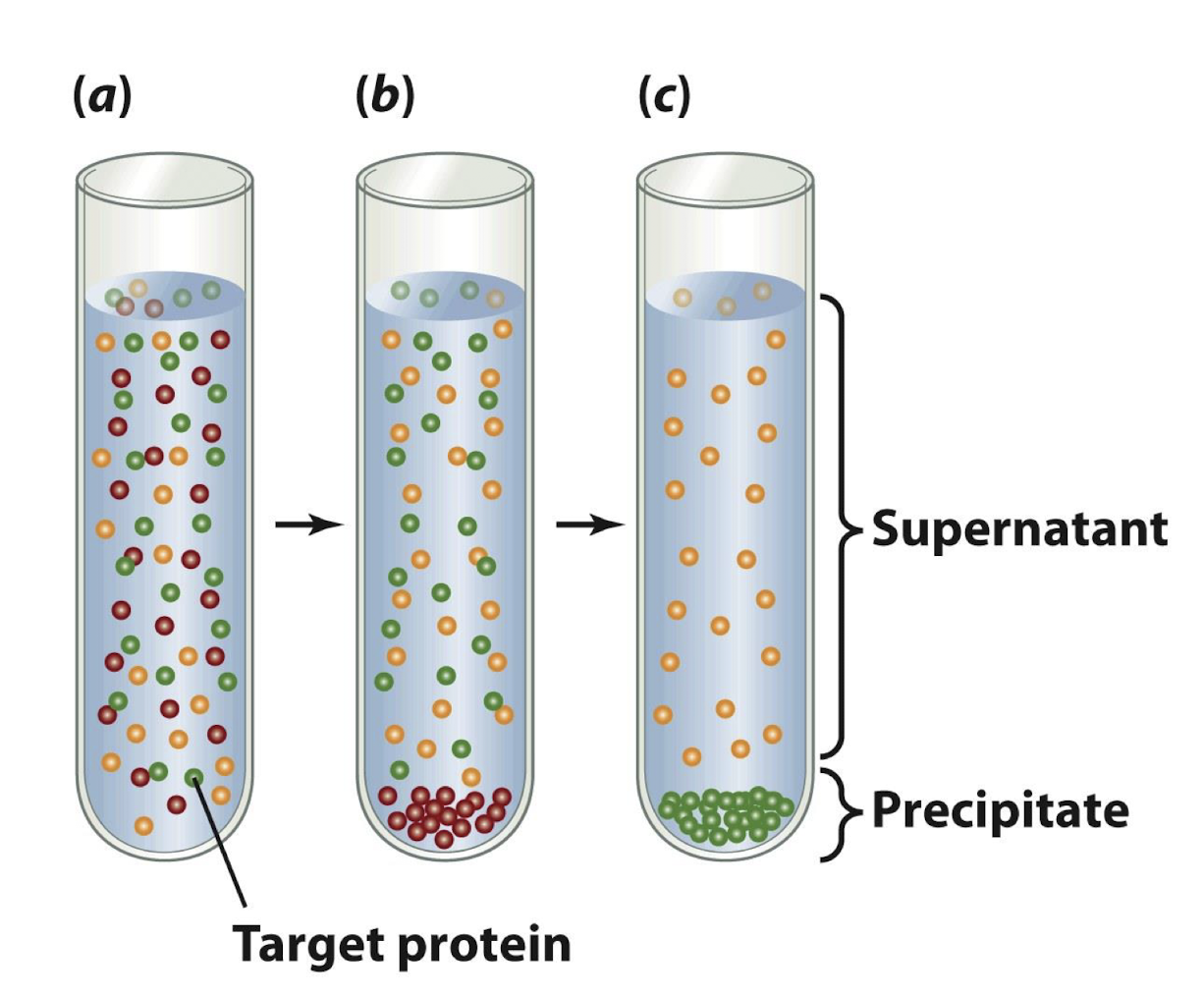
____________ is an example of a salt that may be used in fractionation of proteins in complex mixtures
ammonium sulphate (NH₄)₂SO₄
precipitation point is the calculated __________ of an ______ in solution
The calculated solubility point of an ion in solution (scale/brine stability calculations).
_______ is the liquid on top of material deposited by _______ or _______
supernatant , settling or centrifugation.
what is the 'salting out' in fractionation of proteins based on?
competition between the added salt molecules and the dissolved solutes
size exclusion chromatography is also known as _________
gel filtration chromatography
what does size exclusion chromatography do?
separates proteins based on their size and shape
In size exclusion chromatography, _____ proteins move through the column more ______ because they can't enter the ____ of the gel, while ______ proteins take _____ as they enter the pores.
larger proteins, quickly , the pores, smaller, longer
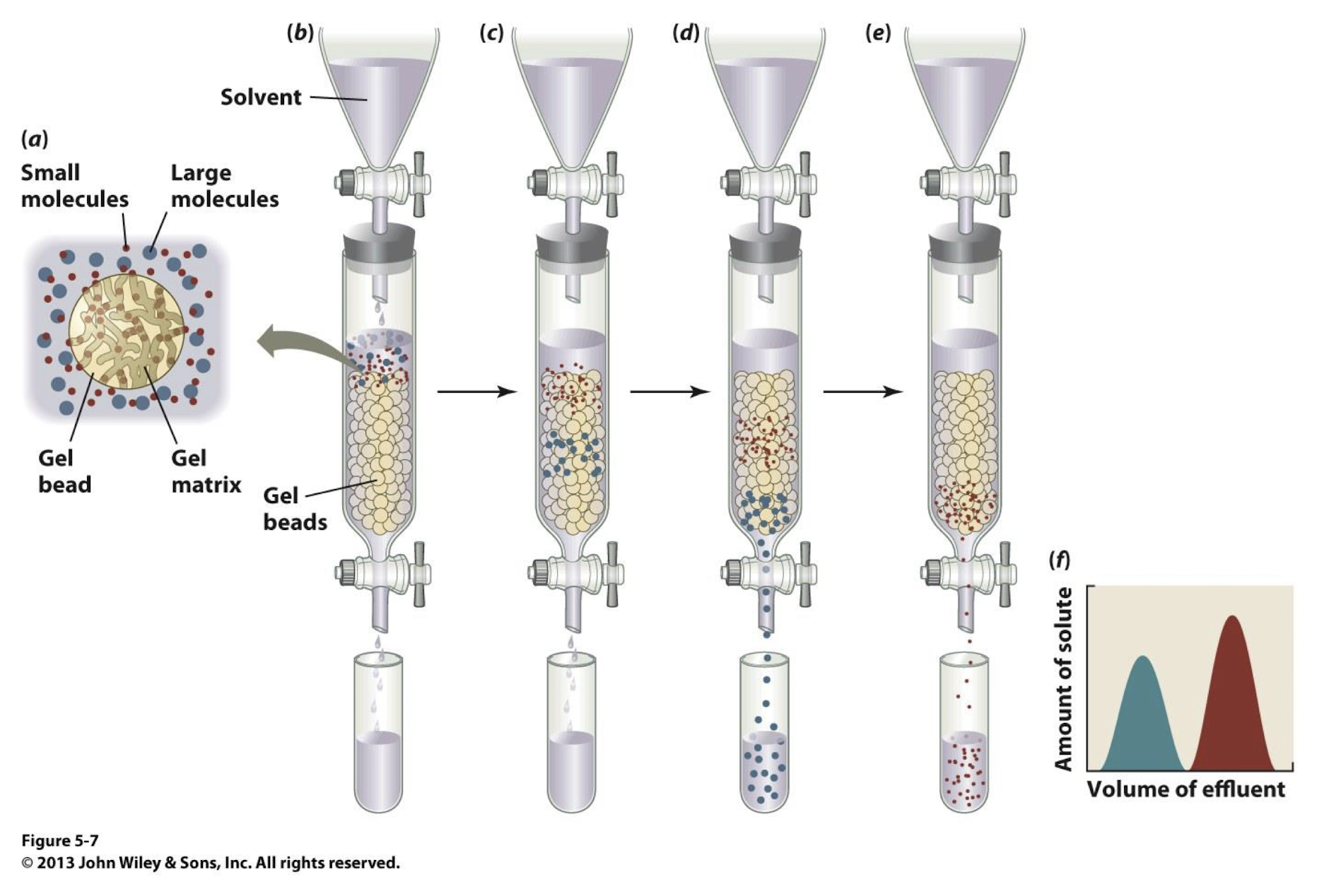
procedure of size exclusion chromatography
The protein mixture is applied to a column filled with a porous matrix.
As the solution flows through the column, proteins are separated based on their molecular size
what does Ion Exchange Chromatography do?
Separates ions and polar molecules based on their affinity to the ion exchanger.
2 types of Ion Exchange Chromatography?
- anion-exchange
- cation-exchange
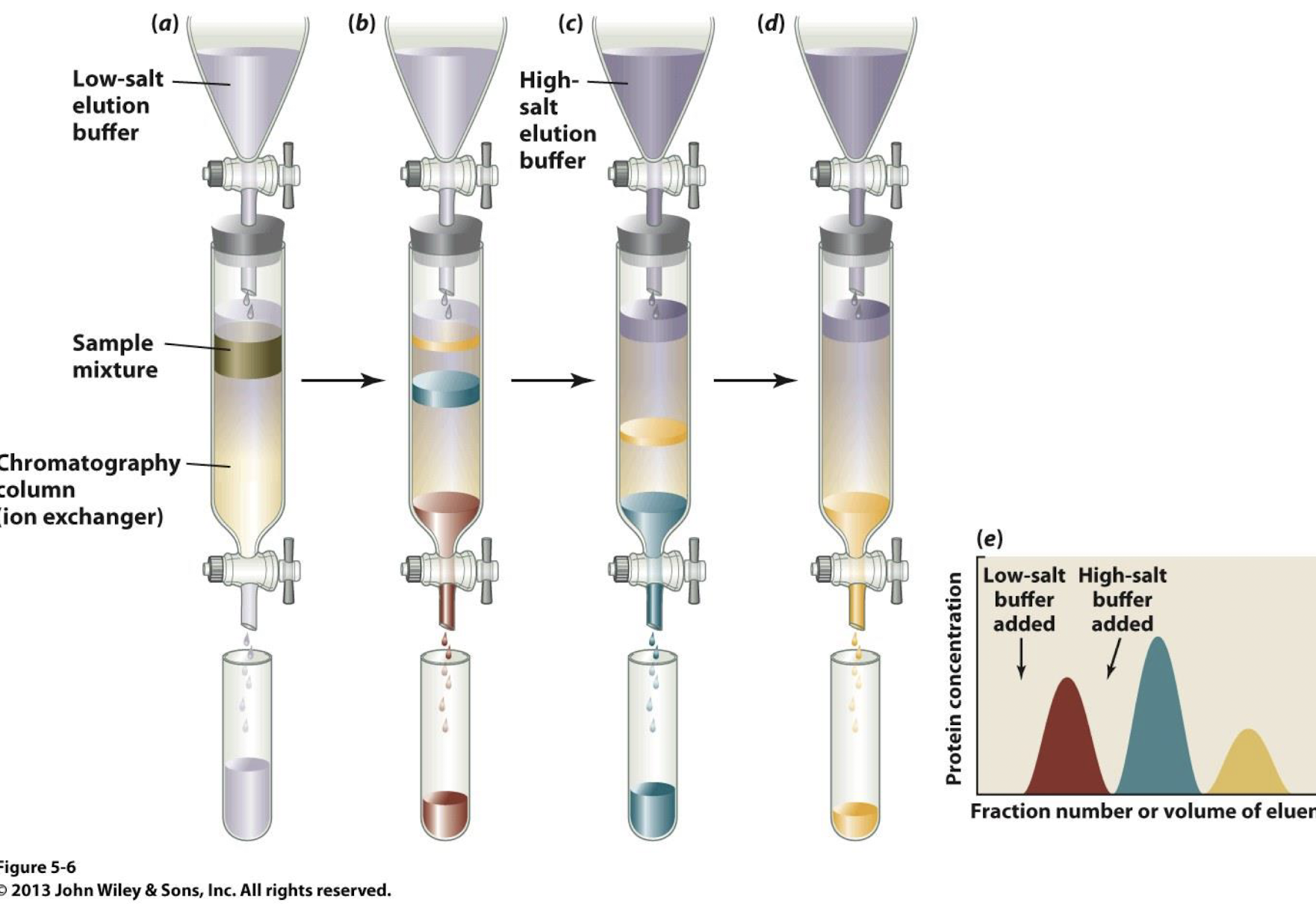
In anion exchange Ion Exchange Chromatography the column resin is _______ charged, attracting _________ charged proteins
positively charged, attracting negatively charged proteins
In cation exchange Ion Exchange Chromatography the column resin is _______ charged, attracting _________ charged proteins
negatively charged, attracting positively charged proteins
principle of Ion Exchange Chromatography:
A charged resin attracts proteins with opposite charges.
what method separates proteins based on their charge?
Ion Exchange Chromatography
what method separates proteins based on their size and shape?
size exclusion chromatography
what method separates proteins based on their binding affinity for to another protein on the column bead?
Affinity Chromatography
what does Affinity Chromatography do?
separates proteins based on their binding affinity for to another protein on the column bead
Affinity chromatography uses a specific interaction between a _____ and a _____ immobilised on a ____
protein and a ligand (eg. antibody enzyme substrate) , matrix
procedure of Affinity Chromatography:
The mixture is applied to a column with the ligand.
The protein of interest binds specifically, while others pass through.
The bound protein is then eluted using a competitive agent.
The most applied affinity system for the purification of antibodies is the _____
Staphylococcal protein A (SPA) and smaller ligands derived thereof
what method separates proteins based on their molecular weight?
Polyacrylamide Gel Electrophoresis
what does the sample buffer for Polyacrylamide Gel Electrophoresis contain?
Sodium dodecyl sulfate (SDS) denatures proteins and confers a negative charge
DTT / β-mercaptoethanol: reducing agents (break disulphide bonds)
what does Sodium dodecyl sulfate (SDS) do in Polyacrylamide Gel Electrophoresis ?
denatures proteins and confers a negative charge
what does DTT / β-mercaptoethanol do in Polyacrylamide Gel Electrophoresis ?
they are reducing agents that break disulphide bonds
principles of Polyacrylamide Gel Electrophoresis
Samples are loaded into wells in a vertical polyacrylamide gel & a current is passed through the gel
Small proteins migrate quicker through the gel, larger proteins stall towards the top of the gel
what is the isoelectric point sometimes. abbreviated as?
pI
The __________ is the pH at which a particular molecule carries _____ electrical charge
isoelectric point , no net
at a proteins pI it is
immobilised in an electrical field
Isoelectric focusing (IEF)
Form of electrophoresis through a stable pH gradient such that a charged molecule migrates to a position corresponding to its isoelectric point.
______ is a a technique that separates particles according to their ____
Mass Spectrometry , mass
Tandem Mass Spectrometry
Peptides are separated by the first MS and one peptide is sent through a collision cell where it collides with helium atoms
5 steps of X-Ray Crystallography
A. Precipitation of a pure preparation of the protein
B. Crystal formation - solid crystal of pure molecule
C. X-Ray diffraction through the crystal -reveals a pattern
D. Capture of the diffraction pattern and rendering by computer software into mathematical co-ordinates
E. Deposition of the 'Crystal Structure' into the database
Cryogenic-Electron Microscopy (cryo-EM) produces
3D images in atomic detail
Proteins in a sample can be quantified by
U.V. spectrophotometry
proteins can be purified according to their
size, solubility, ionic charge or binding properties
Protein sequence can be determined by
mass spectrometry
Protein structure can be determined by
x-ray crystallography or cryogenic-electron microscopy .