Tablets and Capsules specifications workshop
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What is a specification?
A statement about an attribute with which the product must comply to be approved for use
Each attribute will have a separate specification
For a batch of pharmaceutical product to pass, the batch needs to pass ALL specification tests
The characterisation of the flow properties is essential pre-requisites of successful dosage form development. Therefore, what tests would you recommend to evaluate the properties of the powder used in tableting and capsules filling?
Angle of repose
Bulk and tapped density
Compressibility index
Hausner ratio
The angle of repose is one of the general tests performed to evaluate the properties of the powder.
Explain the purpose of this test and the method for performing it.
The angle of repose value indicates the flowability of a powder
A lower angle of repose below 30°) suggests that the powder has good flow properties, meaning it moves easily and uniformly, which is ideal for processes like tabletting and capsule filling
A higher angle (above 40°) reflects poorer flowability, indicating that the powder may be prone to clumping or resistance when flowing, which can lead to issues with consistent dosing and manufacturing
Given that the average height of the powder mix is 4 cm, and the average diameter is 16 cm calculate the angle of repose and comment on it.
Angle of repose = 26.57 = excellent flow
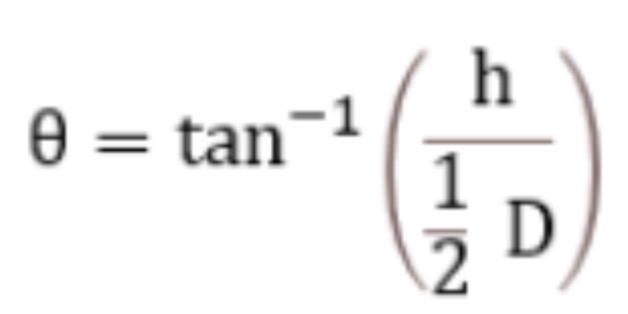
The compressibility index and Hausner ratio are essential tests for assessing the flow and packing characteristics of powders
Explain the purpose of these tests and the method for performing them
Hausner Ratio:
This test provides a ratio that helps assess the flow characteristics of a powder.
A lower Hausner Ratio (close to 1.0) = better flowability
A higher Hausner Ratio (above 1.25) = poorer flow and higher cohesiveness
Carr's (Compressibility) Index
This test measures the powder's ability to reduce in volume when tapped, giving an indication of both flowability and compressibility
A lower Carr's Index (below 15%) suggests good flowability
A higher Carr’s index (above 25%) indicates poor flow and high compressibility, often signalling cohesiveness or the potential for clumping
Method:
Weigh a known quantity of powder (e.g., 30 g) and place it in a graduated cylinder or a specific measuring container
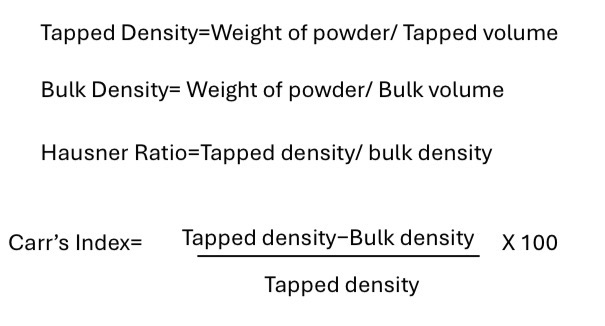
Calculate the bulk density of the powder: weight of powder / bulk volume
Using a tap density apparatus, tap the cylinder containing the powder a specified number of times (usually 100-500 taps, depending on the procedure).
Measure the new volume of the powder after tapping (tapped volume)
Calculate the tapped density: weight of powder / tapped volume
Calculate the Hausner Ratio: tapped density / bulk density
Calculate the Carr’s Index = (tapped density - bulk density) / tapped density x 100
A 30 g powder sample was placed in a tap density apparatus, with a bulk volume of 79 mL and a tapped volume of 62 mL. Calculate the bulk density, tapped density, compressibility index, and Hausner ratio, and provide an interpretation of the results
Bulk Density: 0.38 g/mL
Tapped Density: 0.48 g/mL
Compressibility Index: 20.83% = moderate flowability
Hausner Ratio: 1.26 = moderate flowability
These results suggest that while the powder has acceptable flow properties, it may benefit from additional flow aids to achieve more consistent and efficient processing, particularly if uniformity in capsule or tablet filling is critical
Which excipients can be added to enhance the flowability of the powder?
Glidants
Lubricants
Why is it essential to ensure that tablets comply with weight uniformity specifications?
To ensure that all tablets of a batch have the same weight (within limits):
To ensure that the tablets all contain the same amount of drug
To ensure that the patient gets a reproducible dose of drug
Describe the method for performing tablets weight uniformity test
Weigh individually 20 tablets from the same batch
Calculate mean weight
Batch passes if it complies with the following specification:


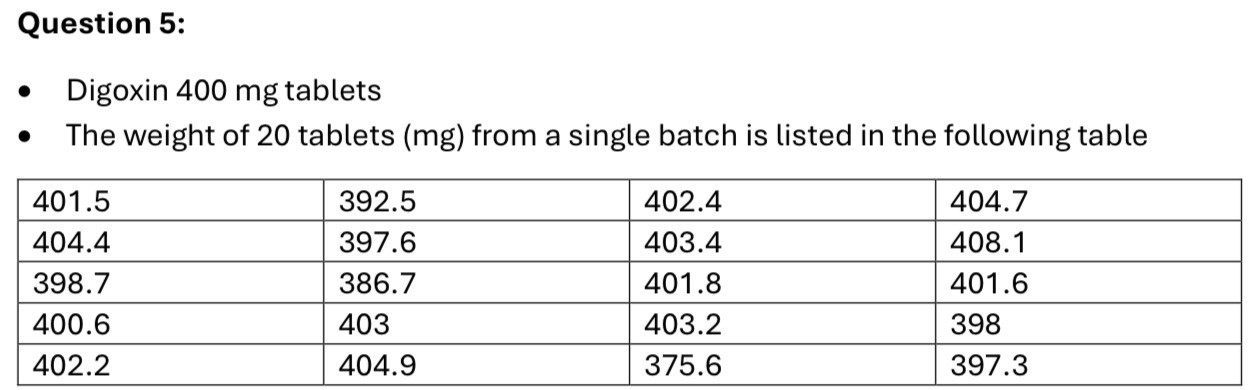
Describe the tablets weight uniformity test and comment on the weight uniformity of this batch of tablets (pass/fail)
Mean = 399.4 mg
Mean ± 5% = 379.4mg to 419.4mg
Mean ± 10% = 359.5mg to 439.4mg
1 tablet outside mean±5% (from the table 375.6)
None outside mean±10%
So the batch passes the weight uniformity test
Describe the method for performing capsules weight uniformity test
Weigh an intact capsule
Empty contents completely
Calculate weight of contents by difference
Repeat 19 times
Calculate mean weight
Describe the capsules weight uniformity test and comment on the weight uniformity of this batch of tablets (pass/fail)
Mean = 399.4 mg
Mean±7.5%: 369.4mg to 429.4mg
Mean±15%: 339.5mg to 459.3mg
Capsules are all within the limits, so the batch passes the weight uniformity test

Friability test was done on a batch of the tablets. The initial weight of the tablets was 7.09 g, and after the test, the weight of the tablets was found to be 7.05 g. What is the purpose of performing a friability test?
To assess the durability and mechanical strength of tablets
It measures the tendency of tablets to break, chip, or crumble under normal handling and transportation conditions
Describe the method for performing a friability test
Weigh a number of tablets (up to 6.5 g).
lace tablets in a drum and rotate for 100 revolutions
Weigh tablets and calculate weight loss
BP specification:
≤1 % weight loss
However, in development 0.2 % weight loss is used as the limit
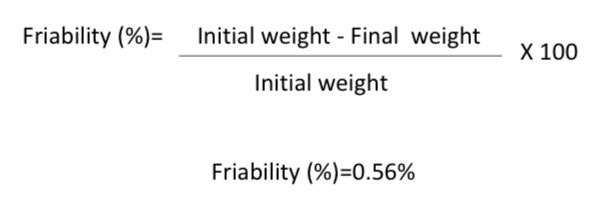
Friability test was done on a batch of the tablets. The initial weight of the tablets was 7.09 g, and after the test, the weight of the tablets was found to be 7.05 g.
What can be concluded about the friability of these tablets according to the given data?
The friability of the tablets is 0.56%, which is below the BP limit of 1%
This indicates that the tablets have good mechanical strength and can withstand handling without excessive breakage or crumbling.
What is the purpose of performing a hardness test?
To ensure that the tablet can withstand handling
To ensure that the disintegration time and dissolution profile remain within specifications (hardness can lead to slower disintegration and dissolution)
Testing "crushing strength"
Hardness is correlated with compaction pressure
Used as an in-process test during compression
Describe the method for performing a hardness test
Select a representative sample of tablets from the batch to be tested. Typically, 10 tablets are tested to ensure uniformity
Use the hardness tester to measure the hardness of the tablets
Calculate the average hardness of the tablets
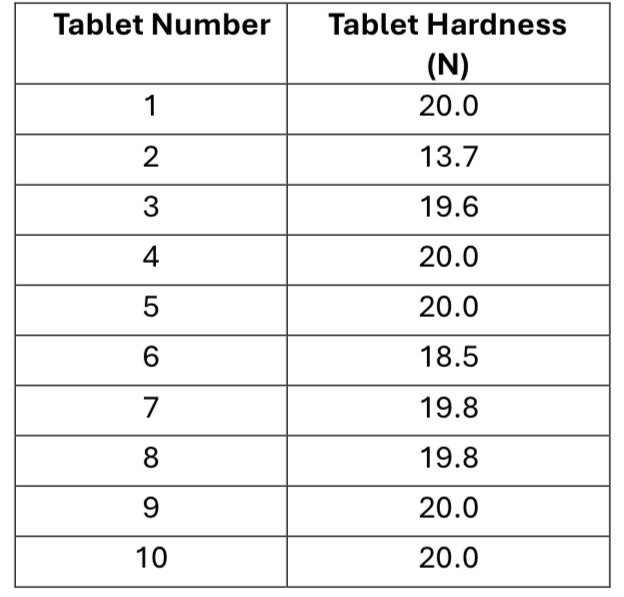
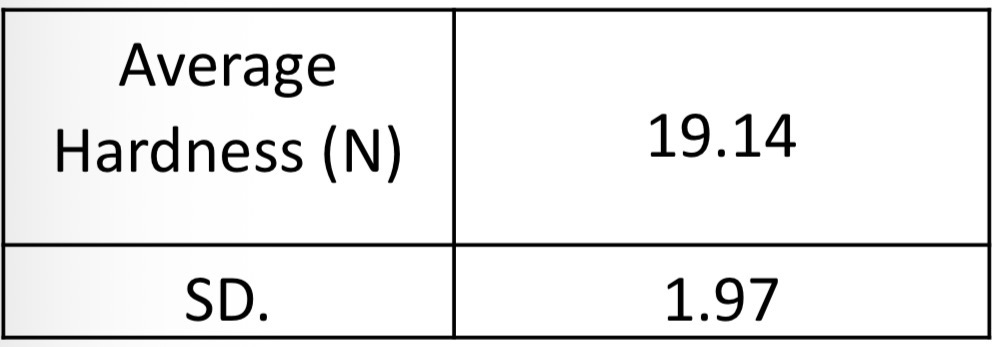
Given that the acceptable range for tablet hardness is 4 N to 12 N for uncoated tablets. What can be concluded about this batch of tablets?
What is the purpose of performing a thickness uniformity test?
Tablet packaging: Uniform thickness ensures that tablets fit properly into blister packs, bottles, or other packaging systems
Mechanical strength: uniform hardness and structural integrity, reducing the risk of tablets, breaking or chipping
Uniform dosage: ensures that the amount of active ingredient in each tablet is uniform
Describe the method for performing a thickness uniformity test
Select a representative sample of tablets from the batch to be tested. Typically, 10 tablets are tested to ensure uniformity.
Use the caliper/hardness tester to measure the thickness of the tablets
Calculate the average thickness of the tablets
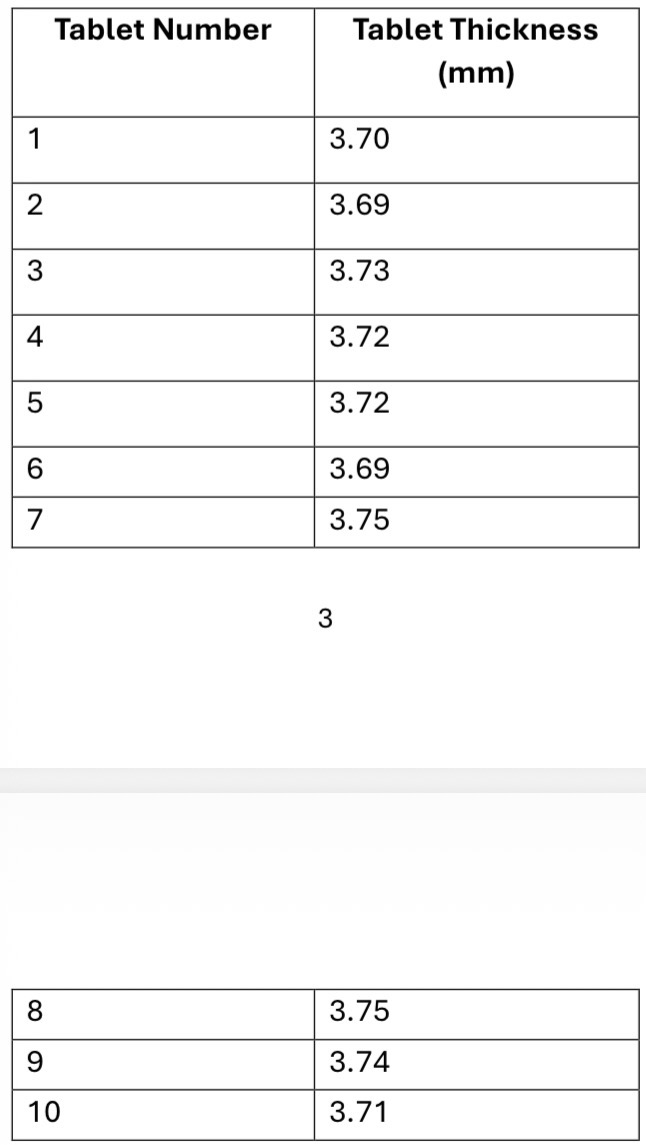
What can be concluded about this batch of tablets?
The general requirement for uniformity of thickness in tablets is usually within ±5% of the average thickness of the sample.
Average thickness = 3.72 mm
SD = 0.02
RSD = SD/mean x 100 = 0.6%
RSD < 5% so the tablets have acceptable thickness uniformity