Chemistry - Periodic Table and Nuclear
0.0(0)
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1
New cards
Alpha Decay
Largest of the nuclear radiation particles
Atomic number DECREASES by 2
Atomic mass DECREASES by 4

2
New cards
Beta Decay
Smaller particles
Atomic number INCREASES by 1
Atomic mass stays the same
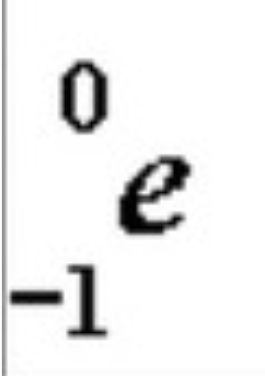
3
New cards
Gamma Emission
wave, not particle
no change in atomic number or mass
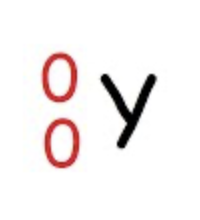
4
New cards
Positron Emission
decreases in the number of protons
Atomic number DECREASES by 1
no change in the mass number
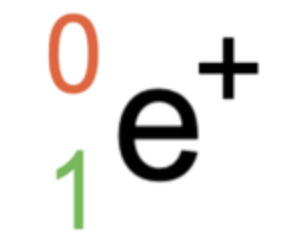
5
New cards
Beta Capture
a proton captures an electron and is transformed into a neutron
Atomic number decreases by 1
no change in the mass