Medic Induction School, Full CPG's Medications.
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
What are the 6 Rights of Drug Administration?
Right Patient
Right Time
Right Dose
Right Route
Right Medication
Right Documentation
What four medications require STT/CSP approval prior to administration? and how do you get approval?
Medications:
Fentanyl (IN)
Fentanyl Citrate (IV)
Intravenous Crystalloid Solution
Olanzapine
How to:
Phone call STT/CSP in comms, give handover/rationale, full a-e observations & justification for requiring the medication.
Adrenaline: (epinephrine)
Introduction:
Presentation: 1mg/1ml (1:1000) ampoule.
Summary: It is a naturally occurring sympathomimetic agent that causes peripheral vasoconstriction. It also stimulates the cardiac conduction system which causes increased contractions along with causing bronchodilation and dilation of blood vessels in the muscles.
IV/IO: Onset 30-seconds, half-life 5-minutes, duration 5-10-minutes.
IM: Onset 60-seconds, half-life 5-minutes, duration 5-10minutes.
Indications: Anaphylaxis, life-threatening asthma, cardiac arrest, post-ROSC, severe croup, haemorrhage control post cricothyroidotomy.
Contraindications: Nil.
Management:
Cardiac Arrest
Adult:
1 mg IV/IO, repeat every 3-5 minutes as clinically indicated
Paediatric / Newborn:
10 microg/kg = 0.1 mL/kg of 1:10,000 adrenaline solution IV/IO, repeat every 3-5 minutes as clinically indicated.
Maximum bolus dose 1 mg (10 mL of 1:10,000 adrenaline solution).
Post-ROSC
Manage hypotension in Post-ROSC patients if BP is slow to rise:
The patient is considered hypotensive if Systolic BP is:
Adult: < 100mmHg
Paediatric: < 80mmHg
Titrate Adrenaline as required, to achieve and / or maintain the SBP requirements as listed above.
Adult:
50 microg (0.5 mL) every 3-5 min as required to maintain systolic blood pressure
Paediatric:
1 microg/kg every 3-5 min (maximum bolus 50 microg (0.5 mL)) to maintain systolic blood pressure
Life-threatening Asthma
Adult:
0.5 mg IM into lateral mid-thigh (0.5 mL of 1:1000), repeat every 5 minutes as clinically required
Paediatric > 1 month:
10 microg/kg IM into lateral mid-thigh (0.01 mL of 1:1000) (maximum single dose = 0.5 mg or 0.5 mL), repeat every 5 minutes as clinically required
Anaphylaxis
Adult:
0.5 mg IM into lateral mid-thigh (0.5 mL of 1:1000), repeat every 5 minutes as clinically required
For impending or actual airway obstruction/stridor/angioedema unresponsive to 2 doses of IM adrenaline:
5 mg (5 mL of undiluted 1:1000 adrenaline) nebulised, single dose only. Consult CSPSOC if further doses required.
The nebulised dose is in addition to ongoing IM doses to manage systemic anaphylaxis symptoms, continue IM adrenaline as required.
Life threatening/peri-arrest* anaphylaxis refractory to 3 doses of IM adrenaline and fluid bolus: 10 microg (0.1 mL of 1:10000) IV/IO every minute as required. CSP consultation recommended prior to administration if possible. If 10 microg doses ineffective after 2 doses contact CSPSOC for higher dosing.
Paediatric > 1 month:
10 microg/kg IM into lateral mid-thigh (0.01 mL of 1:1000) (maximum single dose = 0.5 mg or 0.5 mL), repeat every 5 minutes as clinically required
For impending or actual airway obstruction/stridor/angioedema unresponsive to 2 doses of IM adrenaline:
5 mg (5 mL of undiluted 1:1000 adrenaline) nebulised, single dose only. Consult CSPSOC if further doses required.
The nebulised dose is in addition to ongoing IM doses to manage systemic anaphylaxis symptoms, continue IM adrenaline as required.
Life threatening/peri-arrest* anaphylaxis refractory to 3 doses of IM adrenaline and fluid bolus: 0.2 microg/kg IV/IO (maximum single dose = 10 microg in 0.1 mL), every minute as required.
* For example: altered conscious state, extremely poor perfusion, systolic blood pressure <90mmHg
Croup
Infants (>1 month) / Paediatric:
Nebulise 5 mg (5 mL of 1:1000) undiluted, repeat after 15 minutes if required (repeat once only, further doses require ASMA consult)
Haemorrhage control post cricothyroidotomy
Soak gauze in 1:20,000 adrenaline solution (see preparation) and apply to cricothyroidotomy site using direct pressure.
Special Considerations
Tachyarrhythmias, palpitations
Hypertension
Pupil dilation
Tremor
Anxiety
Precautions/Notes: Ischaemic heart disease, hypertension, hypovolaemia, do not walk patient pre or post IM adrenaline administration in anaphylaxis - usually a minimum of 1-hour after 1 dose of adrenaline and 4-hours if more than one dose of Adrenaline given. If given IV into a peripheral vein, follow each dose with a sodium chloride flush.
Preparation: Cardia Arrest: Paediatric/Newborn - Dilute 1mg in 1ml (1:1000) adrenaline with 9ml sodium chloride 0.9% to produce 1mg in 10ml (1:10,000).
Post-ROSC & Life threatening/peri-arrest anaphylaxis: Dilute 1mg in 1ml (1:1000) adrenaline with 9ml sodium chloride 0.9% to produce 1mg in 10ml (1:10,000).
Haemorrhage control post cricothyroidotomy: Dilute 1mg in 1ml (1:1000) adrenaline with 19ml sodium chloride 0.9% and soak sterile gauze in solution (1:20,000).
Special Considerations:
Tachyarrhythmias, palpitations
Hypertension
Pupil dilation
Tremor
Anxiety
Aspirin:
Introduction:
Aspirin has 4 pharmacological actions: Analgesic, Antipyretic, Anti-inflammatory, Anti-platelet aggregation. It also reduced mortality significantly in Acute Myocardial Infarction by minimising platelet aggregation and thrombus formation in order to retard the progression of coronary artery thrombosis.
Presentation: 300mg white chewable or dispersible tablet.
Indications: Patient with suspected Acute Coronary Syndromes.
Contraindications: Known hypersensitivity to aspirin/salicylates/NSAIDs. Children <16yrs of age.
Precautions/Notes: Actively bleeding peptic ulcers. Suspected AAA. Aspirin/salicylate-sensitive asthmatics.
Management: 300mg oral administration, chewed or dissolved in a small amount of water (depending on presentation). Administered even if patient has take aspirin that day or on anticoagulants.
Special Considerations: Heart burn, nausea, GI bleeding. Increased bleeding time. Anaphylactic reaction (some patients, especially asthmatics) exhibit notable sensitivity to aspirin, which may provoke various hypersensitivity/allergic reactions.
Cophenylcaine:
Introduction: A pump spray containing: Lidocaine (lignocaine) hydrochloride monohydrate 5%, 5mg/spray and Phenylephrine hydrochloride 0.5%, 500microg/spray. It is a topical local anaesthetic and haemorrhage control agent for the relief of surface pain, nasal and bleeding.
Presentation: Spray bottle
Indications: Local pain: abrasions, small cuts and wounds. Relief of mild and moderate epistaxis. Post tonsillectomy haemorrhage. Intra-oral haemorrhage.
Contraindications: Hypersensitivity to phenylephrine, lidocaine or other anaesthesia. Children <2yrs. Pregnancy.
Precautions/Notes: Used with caution is patients with cardiovascular, hepatic and/or renal disease. For oral use, nozzle inserted within the anterior 1/3 of mouth to avoid gag stimulation. Pause between subsequent doses.
Management:
Adult:
Intranasal: Maximum 10 sprays (5 sprays per nostril).
Topically: Maximum 5 sprays.
Oral use: 1 spray then pause, repeat if required up to a maximum of 5 sprays.
Paediatric:
Intranasal: 2-4yrs = 1 spray per nostril. 4-8yrs = 2 sprays per nostril. 8-12yrs = 3 sprays per nostril.
Topically: Maximum 5 sprays.
Oral use: 1 spray the pause, repeat if required up to a maximum of 5 sprays.
Max dosages should not be administered more than once in 24hrs.
Special Considerations: Oral administration may cause a transient bitter taste.
Fentanyl:
Introduction: A short acting synthetic narcotic analgesic.
Presentation:
Fentanyl: 450microg/1.5ml (300microg/ml); intra-nasal administration only.
Fentanyl Citrate: 100microg/2ml ampoule (50microg/ml); IV/IO only.
Fentanyl Citrate: 500microg/10ml ampoule (50microg/ml); IV/IO only.
Indications: Moderate to severe pain. Acute Coronary Syndromes where GTN has been ineffective.
Contraindications: Hypersensitivity to fentanyl. Child <1yr (for IV/IO only). Occluded nasal passages of epistaxis (for IN only).
Precautions/Notes: Elderly patients. Respiratory depression: especially those at risk e.g. patients with severe COPD. Patients currently on MAO inhibitors or MAO inhibitor use within previous 14-days. Caution in larger doses of women in active labour. Use of IV ketamine as analgesic prior to minimum (age dependant) dose of IV fentanyl requires ASMA authorisation:
Paediatric: 100 microg.
Adult <70yrs: 200 microg.
Adult >70 (or frail): 100 microg.
Administer slowly. Cease administration prior to calculated dose if desired effect is obtained. Patients under extended care (e.g. ramped patients) who have already been administered pain relief should have careful consideration with regards to the dosages of fentanyl administered, titrating only to effect.
Preparation: Fentanyl citrate for IV/IO administration: Dilute 100 microg in 2ml with 8ml NaCl 0.9% to produce 10 microg/1ml.
Management:
IV/IO:
Ramped patients must not have loading doses administered - maintenance doses (max 25 microg) to effect as required.
Adult <70yrs old:
Pre-hospital loading dose: titrate 1microg/kg, slow push over 3-5 minutes (max single dose: 100 microg).
Subsequent dose: 25 microg to effect every 5 minutes, titrated to effect.
Adult >70yrs old or frail:
Pre-hospital loading dose: titrate 0.5microg/kg, slow push over 3-5 minutes (max single dose: 50 microg).
Subsequent dose: 25 microg to effect every 5 minutes, titrated to effect.
Paediatric:
Pre-hospital loading dose: titrate 0.5-1microg/kg, slow push over 3-5 minutes (max single dose: 35 microg).
Subsequent dose: 1 microg/kg (up to 25 microg) every 5 minutes, titrated to effect.
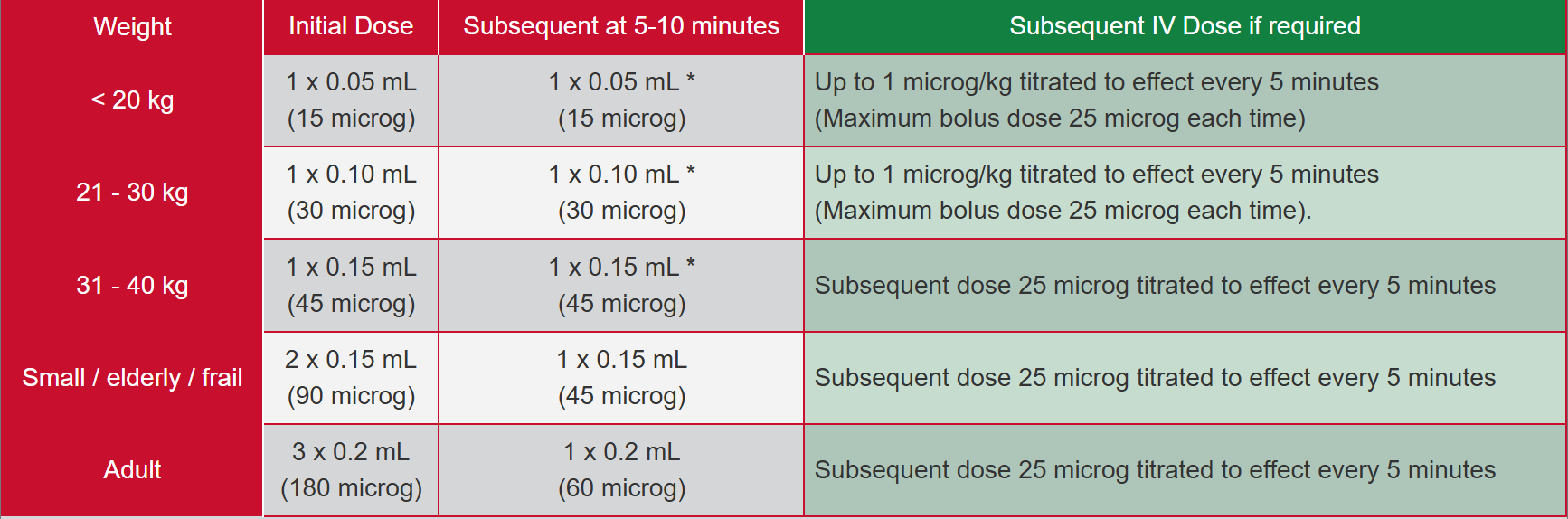
Intranasal:
<20kg:
Initial Dose: 1× 0.05ml (15 microg).
Subsequent at 5-10 mins: 1× 0.05ml (15 microg).
Subsequent IV dose if required: Up to 1 microg/kg titrated to effect, every 5 minutes (maximum bolus dose 25 microg each time)
21-30kg:
Initial Dose: 1× 0.10ml (30 microg).
Subsequent at 5-10 mins: 1× 0.10ml (30 microg).
Subsequent IV dose if required: Up to 1 microg/kg titrated to effect, every 5 minutes (maximum bolus dose 25 microg each time)
31-40kg:
Initial Dose: 1× 0.15ml (45 microg).
Subsequent at 5-10 mins: 1× 0.15ml (45 microg).
Subsequent IV dose if required: Subsequent dose 25 microg titrated to effect every 5 minutes.
Small/elderly/frail:
Initial Dose: 2× 0.15ml (90 microg).
Subsequent at 5-10 mins: 1× 0.15ml (45 microg).
Subsequent IV dose if required: Subsequent dose 25 microg titrated to effect every 5 minutes.
Adult:
Initial Dose: 3× 0.2ml (180 microg).
Subsequent at 5-10 mins: 1× 0.2ml (60 microg).
Subsequent IV dose if required: Subsequent dose 25 microg titrated to effect every 5 minutes.
Subsequent IN dosages can be administered every 5-10minutes, titrated to effect for adult patients. FOR PAEDIATRIC PATIENTS SINGLE REPEAT DOSE ONLY (INITIAL + 1x REPEAT). CSPSCC consult required for doses beyond this in paediatric patients.
Special Considerations: Adopt a low threshold to engage with the ED team if pain remains difficult to control. Drowsiness. Nausea/vomiting. Respiratory depression; monitor pulse oximetry for all patients having IV/IN Fentanyl. Cardiovascular effects: Bradycardia, Hypotension (rare).
Glucagon:
Introduction: A hyperglycaemic agent that increases blood glucose concentration by activating hepatic glucose production and decreasing GI motility.
Onset: 4-7 minutes, duration 10-40 minutes.
Presentation: 1mg in 1ml vial, accompanied by diluent for injection (Hypokit).
Indications: For demonstrated hypoglycaemia where oral glucose cannot be administered and IV access cannot be obtained in a safe and timely manner. Altered conscious state in a known diabetic or of otherwise unknown cause where blood glucose level is below 4mmol/L.
Contraindications: Hypersensitivity. Known pheochromocytoma, insulinoma, glucagonoma.
Precautions/Notes: Glucagon is effective in treating hypoglycaemia only if sufficient liver glycogen is present (i.e. does not work on alcohol or anorexia induced hypoglycaemia). Give complex carbohydrates orally when patient has responded to prevent recurrent hypoglycaemia. Even if fully recovered, patients should be encourage to be transported to a medical facility to ensure effective follow up and review.
Management: Intramuscular injection into deltoid muscle or mid-lateral thigh.
Adult:
1mg (1ml) IM.
Response to glucagon should occur in 10-minutes; if no response, give IV glucose.
If unable to obtain IV access, repeat IM glucagon after 10-minutes if patient still has inadequate GCS and blood glucose level.
Paediatric:
<25kg: 0.5mg (0.5ml) IM.
>25kg: 1mg (1ml) IM.
Single dose only.
Special Considerations: Nausea/vomiting. Gastric pain. Transient rise of blood pressure for patients taking beta blockers.
Glucose Oral Gel:
Introduction: Oral glucose (dextrose) for rapid response to mild hypoglycaemia.
Presentation: Dextrose 15g Oral Gel (Glutose).
Indications: Hypoglycaemia, altered conscious state in known person with diabetes or of unknown medical cause, where blood glucose level is below 4mmol/L. Patient must be able to safely take gel orally/buccally.
Contraindications: Nil.
Precautions/Notes: Monitoring required: Blood glucose level. If unconscious, have patients in lateral position and airway patent. Do not delay transport for paediatric patients. Potential airway obstruction with oral administration, particularly in young children (under 1yr), administer per instructions with caution. PI/AP/Extended care scoped officers: severe hypoglycaemia (loss of consciousness, seizure) consider Glucagon/IV Glucose.
Management:
Oral/Buccal:
Adult: 15g (entire contents of tube), repeat after 10mins if required.
Child: Administer 15g in small amounts to effect, repeat after 10mins if required.
Neonate/Infant: <1yr: Place small amount of gel onto gloved finger and massage into buccal mucosa, titrate to effect.
Special Considerations: Monitoring required post administration: Even if fully recovered, encourage patient to consume a long-acting complex carbohydrate (biscuit, bread) following oral glucose administration to prevent delayed hypoglycaemia.
Glyceryl Trinitrate (GTN):
Introduction: Nitrates cause the relaxation of vascular smooth muscle resulting in:
Vasodilation.
Peripheral pooling and reduced venous return.
Reduced left ventricular end diastolic pressure (preload).
Reduced systemic vascular resistance (afterload).
Reduced myocardial energy and oxygen requirements.
Relaxes spasm of coronary arteries.
Also known as nitroglycerin.
Presentation: Spray bottle containing 200x atomised sprays. 50mg in 10ml for IV infusion.
Indications: Chest pain/discomfort of presumed cardiac origin not relieved by rest and reassurance with:
Systolic BP>90mmHg; AND
Heart rate is between 50-150bpm.
Acute Cardiac Pulmonary Oedema with systolic BP >90mmHg. Autonomic Dysreflexia with systolic BP> 160mmHg.
Contraindications: Hypersensitivity. Hypotension <90mmHg. Ventricular Tachycardia (VT). Recent use of medications used for sexual dysfunction or specific regular medication used in the treatment of pulmonary arterial hypertension (brand names below are not exhaustive:
Sildenafil (Viagra/Revatio), Vardenafil (Levitra) or Avanafil (Spedra) use in the previous 24hrs.
Tadalafil (Cialis) use in the previous 3-days.
Riociguat (Adempas).
Precautions/Notes: Nitrates are an early intervention and should not be delayed until on the stretcher or inside the ambulance. Administer to the patient in a seated or semi-recumbent position. Prime the bottle before using it for the first time by pressing the nozzle 5 times, spraying it into the air. Do not shake GTN bottle prior to administration. Assess BP before every dose. Severe hypotension is an uncommon side effect. Intoxication (effect are enhanced). Phosphodiesterase 5 inhibitor medication administration in previous 4-days.
COVID-19/Febrile Respiratory Illness: Crews should allow the patient to administer their own GTN spray whenever possible. Allow patient to self-administer GTN spray under crew direction; stand clear and wait a minute before approaching the patient. If you have to use SJA supplied GTN spray, assess whether it can reused and wipe with clinell wipe after use. Discard the MDI in the sharps bin if the patient is very unwell or highly symptomatic of infectious respiratory condition. NOTE: If administering St John supplied medication, crew are NOT to lead the remainder of the medication with the patient. This is a violation of the St John WA poisons licence and the Medicines and Poisons Act 2014. No vehicle should be considered non-operational due to not having GTN available; if GTN is not available step up to the next level of pain relief.
Management:
Cardiac Chest Pain:
400microg (1 spray) sublingually.
If pain persists after 5mins and BP maintained, consider further sprays of GTN at 5 minute intervals.
Should the first 3 doses provide some relief but symptoms persist, continue with further doses at 5 minute intervals if no contraindications.
Acute Cardiogenic Pulmonary Oedema:
400microg (1 spray) sublingually.
If BP maintained, consider further sprays of GTN at 5 minute intervals.
Should the first 3 doses provide some relief but symptoms persist, continue with further doses at 5 minute intervals if no contraindications.
Autonomic Dysreflexia:
400microg (1 spray) sublingually.
Repeat doses at 5 minute intervals until symptoms resolve or systolic BP <160mmHg.
Special Considerations:
Side Effects: Hypotension (rare). Tachycardia. Flushing. Headache. Dizziness.
Intravenous Crystalloid Solution (Normal Saline):
Introduction: A sterile isotonic crystalloid solution.
Presentation:
Normal saline (NaCl 0.9%) in 1000ml soft plastic bag.
Normal saline (NaCl 0.9%) in 250ml soft plastic bag.
10ml plastic vial.
Normal saline (NaCl 0.9%) sterile pre-filled 5ml flush syringe.
Indications: Fluid replacement (volume expansion) for the treatment of shock, fluid loss and cardiac arrest.
Contraindications: Severe Pulmonary Oedema.
Precautions/Notes:
Adult patients with penetrating trauma, ectopic pregnancy or aortic aneurysm with hypotension and signs of impaired organ perfusion may benefit from permissive hypotension (systolic blood pressure of 70mmHg).
Fluid therapy for shock, DKA & Hyperosmolar Hyperglycaemic state: Initial fluid therapy is directed toward expansion of the intravascular, interstitial, and intercellular volume, all of which are reduced in hyperglycaemic crises and restoration of renal perfusion.
Management:
KVO:
20 drops per minute (20 drops = 1ml)
Fluid therapy for shock, DKA & Hyperosmolar Hyperglycaemic state:
Adult:
250ml boluses to a max total of 2000ml
Small adult/elderly 250ml boluses up to a max total of 1000ml
Paediatric:
10ml/kg over 5-10 minutes. Repeat once only.
Haemorrhage:
Adult:
Infuse 250ml boluses max total 2000ml with reassessment between each infusion.
Paediatric:
Hypotensive paediatric patients should receive IV fluids; 10ml/kg (max. 350ml bolus) reassessment between each bolus (4x infusions maximum) to a total infusion not exceeding 1000ml.
Cardiac Arrest:
Adult/Paediatric:
20ml/kg bolus as a reversible cause of hypovolaemia.
Newborn:
10ml/kg as a reversible cause of hypovolaemia.
Post ROSC:
Manage hypotension in Post-ROSC patients if BP is slow to ride:
The patients is considered hypotensive if systolic BP is:
Adult: <100mmHg
Paediatric <80mmHg
Adult:
250ml boluses to a maximum total of 500ml with reassessment between each infusion.
Paediatric:
10ml/kg, repeat once only with reassessment between each infusion (bolus max, 250ml).
Burns:
Apply modified parkland formula to patients that meet the following criteria:
Adults:
>15% TBSA.
Paediatrics:
≥ 18mths and >10% TBSA OR
<18mths and >8% TBSA.
Modified Parkland Formula:
2ml x %TBSA x weight of patient.
50% of total amount over first 8 hours.
50% of total amount over next 16 hours.
Special Considerations: Hypervolemia.
Parkland Formula: 4ml x weight in kg x %TBSA burn.
Ipratropium Bromide:
Introduction:
An anticholinergic bronchodilator. It inhibits the vagal reflexes that mediate bronchospasm.
Combined with a nebulised short-acting beat-2 agonist (e.g. salbutamol), ipratropium bromide produces significantly greater bronchodilation than a short-acting beta-2 agonist alone.
Presentation:
250 microg/1 mL nebule
Metered Dose Inhaler (MDI)
20 microg per puff
Indications: Severe Bronchospasm:
Adult:
Severe to life-threatening asthma or COPD.
Paediatric:
Severe to life-threatening asthma.
Contraindications: Hypersensitivity.
Precautions/Notes:
Glaucoma.
Avoid contact with eyes.
COVID-19/Febrile Respiratory Illness:
Please review guidance on Nebulisers.
Crews should allow the patient to administer their own Ipratropium Bromide MDI via spacer whenever possible.
Allow patient to self-administer Ipratropium Bromide per their asthma management plan under crew direction; stand clear and wait a minute before approaching the patient.
If you have to use SJA supplied Ipratropium Bromide MDI, assess whether it can be reused and wipe with Clinell wipe after use. Discard the MDI in the sharps bin if the patient is very unwell or highly symptomatic of infectious respiratory condition.
Note: If administering St John supplied medication, crews are NOT to leave the remainder of the medication with the patient. This is a violation of the St John WA poisons licence and the Medicines and Poisons Act 2014.
Crews may tolerate lower oxygen saturations in patients with infective respiratory symptoms prior to considering intervention, as the use of MDI’s may precipitate a cough. See Oxygen Delivery for specifics regarding SpO2 tolerance.
Management:
Adult:
Nebulised (combined with salbutamol):
500 microg in 2 mL (250 microg/mL)
Dilute solution for nebulisation to 2-3ml with sodium chloride 0.9%
Repeat nebulised dose every 20mins; maximum of 3 doses.
MDI:
8 puffs (160 microg), 1 breath per puff - if possible, give via spacer
Repeat MDI dose every 20 minutes; maximum of 3 doses.
Paediatric:
Nebulised:
< 6yrs: 250 microg in 1ml.
> 6yrs: 500 microg in 2ml.
Subsequent dose if required: Repeat nebulised dose every 20mins; max of 3 doses.
Notes: Dilute solution for nebulisation to 2-3ml with sodium chloride 0.9%.
MDI:
< 6yrs: 4 puffs (80 microg).
> 6yrs: 8 puffs (160 microg).
Subsequent dose if required: Repeat MDO dose every 20mins; max of 3 doses.
Notes: Give via spacer.
Special Considerations:
Headache.
250 microg/1 mL nebule
Metered Dose Inhaler (MDI)
20 microg per puffNausea, dizziness.
Dry mouth, throat irritation.
Taste disturbance.
Skin rash.
Loratadine:
Introduction: Second generation (less-sedating) long acting antihistamine.
Presentation: 10mg tablet.
Indications: Symptomatic urticaria (without evidence of anaphylaxis).
Contraindications:
Children < 30 kg.
Hypersensitivity to loratadine.
Precautions/Notes:
Severe hepatic impairment.
Elderly: risk of sedation and anticholinergic effects increased.
Not to be administered within 24 hours of previous antihistamine dose without ASMA approval.
Antihistamines have no role in the treatment or prevention of respiratory or cardiovascular symptoms in acute anaphylaxis.
Management: Adult and Child > 30 kg: 10 mg tablet as single dose only.
Special Considerations: Common Adverse Effects: drowsiness, fatigue, headache, nausea, dry mouth.
Methoxyflurane:
Introduction:
Inhaled anaesthetic.
Onset of pain relief after 6-10 inhalations.
Pain relief lasts 20-30 mins with continuous use, up to 60 mins with intermittent use.
Presentation: Penthrox® 3ml vial for inhalation.
Indications: Analgesia.
Contraindications:
Hypersensitivity to fluorinated anaesthetics.
Children under 1 year of age.
Patients who are unable to understand or co-operate including those affected by alcohol or illicit drugs.
Patients with a severe head injury and altered state of consciousness.
Patients susceptible to malignant hyperthermia.
Precautions / Notes:
Monitoring required: Monitor for over-sedation and apneoa, particularly in children under 5.
Equipment required: Penthrox® Inhaler Device with charcoal filter attached.
Renal impairment: renal toxicity in high doses. May worsen declining renal function.
Children under 5 may require assistance using the device.
Elderly: possible reduction in blood pressure or heart rate.
Management:
Inhalation
Adult/Paediatric > 1 year old:
3 mL vaporized in a Penthrox® Inhaler device inhaled intermittently to maintain adequate analgesia.
Repeat if necessary after 30 mins up to a total of 6 mL daily.
Maximum of 5 doses (15 mL) per week.
Not recommended to use on consecutive days.
Special Considerations:
Common Adverse Effects: cough (initial dose, advise to inhale gently), dizziness, drowsiness, headache, dry mouth, disinhibition.
Monitoring required post administration: Pain score
Naloxone:
Introduction: Naloxone is a pure opioid antagonist that exerts its effect by competitive inhibition at the opioid receptor sites. It prevents or reverses the effects of opioids, including respiratory depression, sedation and hypotension. In the absence of opioids, it exhibits essentially no pharmacological activity.
Presentation: 0.4mg (400 microg) in 1ml vial.
Indications: Reversal of respiratory depression in a suspected narcotic overdose.
Contraindications: Hypersensitivity to Naloxone.
Precautions / Notes:
Polypharmacy overdose.
Half-life of naloxone is < 1 hour; repeat doses may be required to maintain effect with longer acting opioids and those with active metabolites (e.g. methadone, diphenoxylate, codeine). Observe patients who respond to naloxone for 2-3 hours after administration for signs of re-narcotisation.
Response to Naloxone is rapid; reconsider diagnosis if there is a failure to respond to 2 mg Naloxone.
Patients may be aggressive post Naloxone and administration due to hypoxia. Scene safety and personal safety are paramount.
IN naloxone is only for EMT scope only, unless no other routes available. For more information, see here.
Preparation:
IM:
Do NOT dilute in general
IV / IO:
Dilute 400microg in 1mL with 9mL NaCl 0.9% to produce 40microg/1mL
Management:
Adult:
IM/IV/IO:
0.4-0.8 mg (400-800 microg) repeat dose every 2 minutes as required, titrated to clinical response to a maximum of 10 mg.
IN:
One spray (1.8 mg) of Nyxoid® into nostril.
Repeat at 2 minutes in opposite nostril if the patient does not respond.
Paediatric:
IM/IV/IO:
0.01 mg/kg (10 microg/kg, maximum dose 0.4 mg (400 microg), repeat every 2-3 minutes minutes as required, titrated to clinical response.
If high suspicion of overdose, a 0.1 mg/kg (100 microg/kg) dose (maximum 2 mg) may be given following a lack of response to initial dose.
IN:
One spray (1.8mg) of Nyxoid® into nostril.
Repeat dose after 2 minutes if patient does not respond.
Special Considerations:
Withdrawal symptoms such as:
Aggression
Agitation
Nausea/vomiting
Dilated pupils and lacrimation
Take-Home-Naloxone (THN)
Total dispensed amount (3.6 mg - 2x 1.8 mg Sprays + Box with Instructions)
For patients that have required Naloxone, every attempt should be made to convince the 'at risk' patient to be transported to a healthcare facility.
Where attempts prove futile, IN Naloxone - Nyxoid® could be left with the patient. This is to avoid the risk of opioid related respiratory depression due to the short half-life of Naloxone.
In such circumstances please ensure to document leaving Nyxoid® with the person or a responsible other person in the PnT / ePCR.
Due to our SASA requirements, clinicians cannot supply scheduled medicines to persons who are not St John WA patients.
Olanzapine Oral Dispersible:
Introduction:
Olanzapine is a second generation antipsychotic agent that acts on multiple receptors (incl. serotonin and dopamine receptors), resulting in sedation
Onset of effect usually ~ 10 mins.
Use of a sedative agent should never be considered routine. Have a high threshold to offer or administer.
Presentation: 5mg Oral dispersible tablet.
Indications:
Disturbed and Abnormal Behaviour (RASS 1 ~ 3) if considered appropriate where risk to safety is evident and de-escalation has not been effective
Patient is able to tolerate or self-administer an oral wafer.
Preferred first line sedation agent in frail patients and those with Dementia.
Contraindications:
Known Allergy.
Known Parkinson’s Disease.
Age < 6 years old.
Precautions / Notes:
Address organic causes for behavioural presentations at all times- eg. CVA, TBI, Hypoxia, Hypoglycaemia, etc.
Dementia patients – apply caution. Use lower doses.
Oral dispersible tablet may be dissolved in water (may slightly delay onset of action but still preferable in non-emergent cases).
‘Agitated or Excited Delirium’, ‘Acute Behavioural Disturbance’ and ‘Drug Induced Psychosis’ are some alternative terms that may be used by other agencies
Sedation Warnings:
Sedation is HIGH RISK – must only be carried out after careful deliberation between officers and must not be based primarily at the request or influence of other agencies on scene (e.g. Police etc.).
Positive RASS score does not automatically infer a need to sedate.
Age <16 years old – sedation should prompt a prior ASMA consult wherever practicable.
Pregnant patients - STORC consult MUST be initiated prior to the decision to sedate in all cases where the patient is known or suspected to be pregnant. Transport of pregnant patients should be in the lateral position to prevent supine hypotension syndrome in any woman greater than 20 weeks gestation and to assist in managing airway secretions.
ETOH / Intoxication – apply caution.
Repeat & Maintenance doses – have a low threshold to consult with ASMA where repeat or maintenance doses are required.
Monitoring – SpO2 and EtCO2 monitoring must be applied whenever level of consciousness drops (~RASS -2 or below).
Positioning – DO NOT transport in supine position (increases risk of laryngospasm from secretions) – transport in lateral position.
Airway & Breathing – monitor airway and breathing effort, including chest movement closely for signs of impairment. Prepare to support if required.
Restraint – Prone and/or handcuffed to rear carries excessive risk and MUST NOT occur. Physical restraint in any position that amplifies the risk of positional asphyxia, must be closely observed for signs of air hunger and hypoxia.
RASS scores must be agreed and documented.
Weight – Estimated weight must be agreed before administration of any weight based medicines. This must be documented.
The final decision to sedate lies with the most senior clinician on scene.
Management:
Adults < 70 years old:
10 mg.
Repeat as necessary after 15 mins to maximum cumulative dose 20 mg/24 hrs (via all routes).
Adults > 70 years old or frail:
5 mg.
Repeat as necessary after 15 mins to maximum cumulative dose 10 mg/24 hrs (via all routes).
Paediatric 6 - 15 years old, > 40kg:
5 - 10 mg.
Repeat as necessary after 15 mins to maximum cumulative dose 20 mg/24 hrs (via all routes).
Paediatric < 40kg
ASMA consult required.
Special Considerations:
Extrapyramidal effects / Dyskinesia.
Increased falls risk.
Hypotension – Apply monitoring as soon as practicable.
Ondansetron:
Introduction:
Antiemetic. Central and peripheral 5HT3 antagonist.
Oral:
Onset of Action: 15 - 30 mins; Half-life: 4 - 11 hrs; Duration of Action: 4 - 8 hrs
IM:
Onset of Action: 10 - 15 mins; Half-life: 2.5 - 6 hrs; Duration of Action: 4 - 8 hrs
IV:
Onset of Action: 3 - 5 mins; Half-life: 2.5 - 6 hrs; Duration of Action: 4 - 8 hrs
Presentation:
4mg wafer.
4mg in 2ml ampoule.
Indications:
Moderate to severe nausea.
Active vomiting.
Nausea and vomiting prophylaxis for eye and spinal injuries.
Contraindications:
Hypersensitivity to ondansetron.
Treatment with apomorphine: risk of severe hypotension and loss of consciousness.
Paediatrics less than 2 years old.
Precautions / Notes:
Monitoring Required: ECG monitoring may be required for IV administration of high or repeated doses in patients with prolonged QT or risk factors for QT interval prolongation.
Serotonin Syndrome: Risk of Serotonin Syndrome in concomitant use of other serotonergic drugs (e.g. SSRIs and SNRIs).
Hepatic Impairment: reduces clearance and prolongs half life.
Phenylketonuria: wafers contain aspartame, administer with caution.
Pregnancy, First Trimester: not recommended, seek advice from CSPSCC/STORC.
Children with gastroenteritis with diarrhoea as the prominent symptom: ondansetron may worsen diarrhoea.
Rapid injection may cause dizziness and transient visual disturbances.
Preparation:
IV/IO Injection: Dilute 4 mg (2 mL) in 8 mL of sodium chloride 0.9% to produce 0.4 mg/mL solution (4 mg/10 mL).
Management:
Oral
Adult:
4 mg oral wafer.
If the patient remains symptomatic, a second dose may be given after 15 minutes.
Ensure that the total dose does not exceed 8 mg within an 8-hour period.
Paediatric (>4 years or >15kg):
4 mg oral wafer, not repeated.
IM injection:
Adult:
4 mg IM injection.
Can be repeated once if patient remains symptomatic after 15 minutes.
No further doses within 8 hrs of second dose.
Paediatric (>2 years):
0.1 mg/kg up to 4 mg as a single dose, not repeated within 8 hrs.
IV/IO injection
Adult:
4 mg over at least 30 secs but preferably 3-5 mins.
If patient remains symptomatic a second dose may be given after 15 minutes.
No further doses within 8 hrs.
Note: Over 75 years: inject slowly as more susceptible to side effects.
Paediatric: (>2 years):
0.1 mg/kg up to 4 mg as a single dose administered over 3-5 mins, not repeated within 8 hours.
Note: IV ondansetron should only be given to patients unable to tolerate oral administration.
Special Considerations:
Common Adverse Effects: constipation, headache, dizziness.
IV: blurred vision (transient visual disturbance with rapid IV administration), dizziness, flushing
Monitoring required post administration: ECG changes following IV administration.
Look alike, sound alike (LASA) medication: Olanzapine orally disintegrating tablet.
Oxygen:
Introduction: Oxygen is a treatment for hypoxaemia and has not been shown to have any effect on breathlessness in non-hypoxaemic patients.
Presentation:
"C" size cylinder: 490 litres.
"D" size cylinder: 1640 litres.
Indications:
Adult:
Oxygen should be titrated to achieve oxygen saturations of between 94 – 98%, (or 88 – 92% for COPD patients). These are achieved through the use of different flow rates and oxygen masks.
Paediatric:
All paediatric patients with significant illness or injury should receive oxygen. Newborn resuscitation should be commenced with room air for the 30 seconds of initial inflation breaths.
Contraindications:
Explosive or flammable environments.
Normoxia.
Precautions / Notes:
If the target saturations cannot be maintained with the nasal cannula or medium concentration mask then change to a non-rebreather oxygen mask.
Oxygen increases the toxicity in paraquat poisoning, target saturations of 88–92%.
Remember that some conditions can affect SpO2 readings e.g. carbon monoxide poisoning and cold digits.
COVID-19 / Febrile Respiratory Illness:
Please review guidance on Nebulisers
Crews should allow the patient to administer their own Salbutamol MDI via spacer wherever possible
Allow patient to self-administer Salbutamol per their asthma management plan or under crew direction; stand clear and wait a minute before approaching the patient
If you have to use SJA supplied Salbutamol MDI, assess whether it can be reused and wipe with Clinell wipe after use. Discard the MDI in the sharps bin if the patient is very unwell or highly symptomatic of infectious respiratory condition.
Note: If administering St John supplied medication, crews are NOT to leave the remainder of the medication with the patient. This is a violation of the St John WA poisons licence and the Medicines and Poisons Act 2014.
Crews may tolerate lower oxygen saturations in patients with infective respiratory symptoms prior to considering intervention, as the use of MDI’s may precipitate a cough. See Oxygen for specifics regarding SpO2 tolerance and Oxygen Delivery for COVID-19 precautions.
Management:
Aim for target saturations of between 94 – 98% for critical conditions requiring supplemental oxygen, maintained via bag-valve-mask or reservoir bag.
In patients with COPD or other conditions requiring controlled or low-dose supplemental oxygen aim for target oxygen saturations range of 88 – 92% (or the patient’s prescribed range).
If the patient is hypoxaemic, oxygen saturations of between 94 – 98% should be maintained through the use of a mask or nasal cannulae as appropriate.
At the correct flow rate the following devices will deliver the following approximate FiO2:
Nasal Cannulae:
Fraction of Inspired O2 (FiO2): 24-25%.
Flow-Rate: 1-4 litres per minute.
Simple face Mask:
Fraction of Inspired O2 (FiO2): 40-60%.
Flow-Rate: 5-8 litres per minute.
Non-rebreather Mask:
Fraction of Inspired O2 (FiO2): 60-100%.
Flow-Rate: 10-15 litres per minute.
Bag-valve Mask:
Fraction of Inspired O2 (FiO2): 100%.
Flow-Rate: 15 litres per minute.
Special Considerations:
Patients with acute episodes of COPD are at risk of developing carbon dioxide retention if they are given excessive supplemental oxygen. This can cause acidosis and subsequent organ dysfunction.
High oxygen concentrations can lead to increased production of reactive free radicals resulting in cellular damage. This may be responsible for the detrimental effects observed with the use of high flow oxygen in myocardial infarction and stroke.
Note: The oxygen required includes a safety margin (100% increase) to cover the oxygen need for loading, diversion, delays and increases in oxygen requirement during patient management/transfer. Crews should ensure they have sufficient oxygen before departing to allow for a safe transport of the patient.
Paracetamol (Acetaminophen):
Introduction:
Analgesic and antipyretic.
Oral:
Onset of action: 30-60 minutes; Half-life: 2 hours; Duration of action: 3-4 hours.
IV:
Onset of action: 5-10 minutes; Half-life: 1-3 hours; Duration of action: 4-6 hours.
Presentation:
500mg tablets.
120mg chewable tablets (Childrens Chewable Panadol).
100mg/mL (2000mg/20ml) oral suspension.
10mg/mL (1000mg/100mL) infusion.
Indications:
Mild to moderate pain.
E.g. Headache, sprain, strain.
As a component of a multimodal analgesic regime.
Contraindications:
Hypersensitivity to paracetamol.
Patients less than 6 months of age (oral solution).
Patients less than 3 years of age 120mg (chewable tablets).
Patients less then 9 years of age (500mg tablets).
Patients less than 1 month of age (IV).
Any paracetamol containing product within the last four hours (including prior to SJWA arrival).
Precautions / Notes:
Paracetamol oral suspension is for single patient use only.
Use syringe/dropper supplied with product unless otherwise directed.
Management:
Oral
Adult: 500 - 1000 mg (1-2 tablets).
May be repeated every 4 - 6 hours, maximum of 4 g (8 tablets) per day.
Paediatric:
For all presentations of product:
May be repeated every 4 - 6 hours.
Maximum of 60 mg/kg per day.
Oral solution:
15 mg/kg2
Oral solution is not for children < 6 months of age.
120 mg chewable tablets:
Not for children < 3 years of age.
3-6yrs Dose: 2 tablets (240mg).
6-9yrs Dose: 3 tablets (360mg).
500mg tablets (for children aged between 9-12 years of age):
9-12yrs Dose: 0.5 to 1 tablets (250-500mg).
IV Infusion:
Adult:
1 g infused over 15 - 20 minutes.
May be repeated every 4 - 6 hours.
Paediatric (> 1 month old):
15 mg/kg infused over 15 - 20 minutes. Maximum of 1000 mg per dose.
May be repeated every 4 - 6 hours.
Special Considerations: Common Adverse Effects: Nausea.
Salbutamol Sulfate:
Introduction:
Short acting Beta 2 agonist that causes relaxation of bronchial smooth muscle (bronchodilation).
Onset: 2-5 minutes, maximum by 10 minutes.
Presentation:
Salbutamol nebules
5 mg in 2.5 mL.Metered Dose Inhaler (MDI)
100 microg per puff.
Indications:
Bronchospasm and respiratory distress associated with wheeze:
Acute Bronchial Asthma
Bronchitis
Smoke inhalation
Severe allergic / anaphylactic reactions
Acute Pulmonary Oedema of non-cardiac origin
Salt Water Aspiration Syndrome (SCUBA divers)
Chronic Obstructive Pulmonary Disease (COPD)
Contraindications:
Known hypersensitivity to salbutamol.
Cardiogenic pulmonary oedema.
Age <12 months.
Precautions / Notes:
A spacer / MDI is the preferred route for salbutamol administration where the patient presents with influenza like illness.
The use of a Metered Dose Inhaler (MDI) and spacer is equally as effective as nebulisation, in all asthma situations, where the patient is still able to adequately inhale.
Use of a nebuliser is recommended where the patient loses this ability.
Ambulance Transport Officers (ATO) are only authorised to use salbutamol MDI in a known asthmatic patient with respiratory distress.
If hypoxic, nebulise salbutamol in preference to MDI, to address both hypoxia and bronchospasm. The nebulised route also makes it possible to administer Ipratropium Bromide simultaneously.
COVID-19 / Febrile Respiratory Illness:
Please review guidance on Nebulisers
Crews should allow the patient to administer their own Salbutamol MDI via spacer wherever possible
Allow patient to self-administer Salbutamol per their asthma management plan or under crew direction; stand clear and wait a minute before approaching the patient
If you have to use SJA supplied Salbutamol MDI, assess whether it can be reused and wipe with Clinell wipe after use. Discard the MDI in the sharps bin if the patient is very unwell or highly symptomatic of infectious respiratory condition.
Note: If administering St John supplied medication, crews are NOT to leave the remainder of the medication with the patient. This is a violation of the St John WA poisons licence and the Medicines and Poisons Act 2014.
Crews may tolerate lower oxygen saturations in patients with infective respiratory symptoms prior to considering intervention, as the use of MDI’s may precipitate a cough. See Oxygen for specifics regarding SpO2 tolerance and Oxygen Delivery for COVID-19 precautions.
Management:
MDI / Space chamber as per Clinical Skill.
Adult/Child > 6 years:
4-12 puffs (400-1200 microg), repeat every 20 minutes (or sooner if needed) for the first hour.
Paediatric < 6 years:
2-6 puffs (200-600 microg), repeat every 20 minutes (or sooner if needed) for the first hour.
Using an MDI with spacer:
Press once firmly on the MDI to discharge 1 puff into the spacer.
Instruct the patient to breathe in and out normally for 4 breaths.
Repeat 1 puff at a time until the appropriate number of puffs have been taken.
Repeat as clinically required as per dosing schedule above.
Nebulised as per Clinical Skill:
Use 1-2 nebules (5-10 mg in 2.5-5 mL) with 6-8 L/min oxygen in a nebuliser mask.
Give salbutamol via continuous nebulisation in life threatening asthma.
Repeat as clinically required.
Special Considerations:
Muscle tremor.
Tachycardia, palpitations.
Headache.