Chemical Equilibrium
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
What is chemical equilibrium
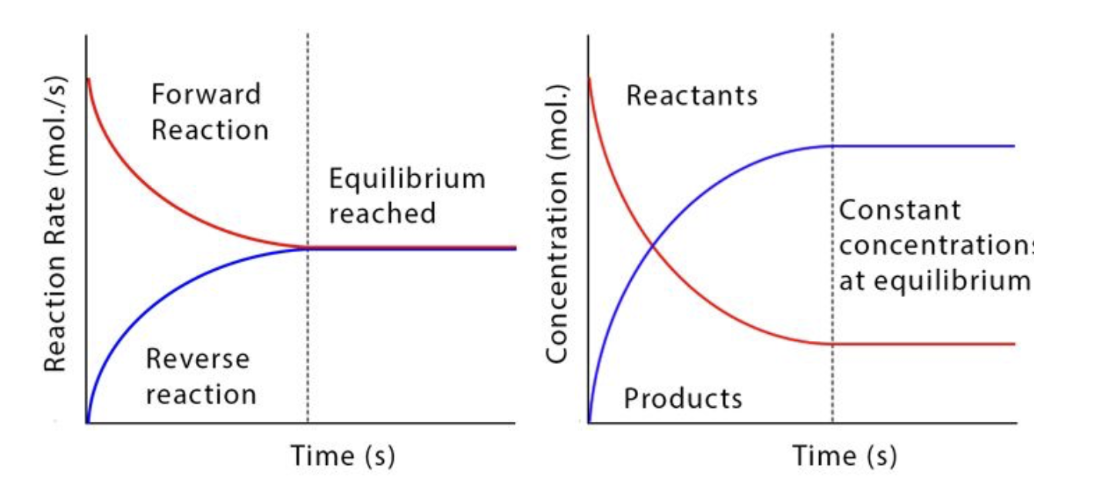
A reaction where the forward and reverse reactions are occurring at the same rate and the concentrations of all reagents are stable
What are the differences between reversible and irreversible reactions?
Reversible → can go forwards and backwards
Irreversible → can only go forwards
Reversible → does not go into completion, so yield is never 100%
Irreversible → reaction continues until the limiting reagent is used up and the reaction goes into completion
Examples of reversible reactions
H2 (g) + I2 (g) ⇌ 2HI [formation and decomposition]
Haber process
N2O4 (g) ⇌ 2NO2 [decompostion and dimerization]
Examples of irreversible reactions
Precipitation
Combustion
Differences between dynamic and static equilibrium
Dynamic → reversible reaction
Static → irreversible reaction
Dynamic → reactants are products still participating in chemical reactions
Static → no further chemical reaction in the system
Dynamic → forward and backward reaction rates are equal
Static → forward and backward reaction rates are 0
Dynamic → only occurs in closed systems
Static → occurs in both open and closed systems
Difference between closed and open system
Open: can exchange both matter and energy with its surroundings
Closed: can only exchange energy, not matter
What is a similarity between irreversible and reversible reactions?
Concentration of reactant and products in total remains constant, though the concentration of reactant as compared to products is different
Graphs of dynamic equilibrium in a closed system
What is Le Châtelier’s Principle?
When a system in dynamic equilibrium is subjected to a change in conditions which disturb the equilibrium, the system will respond in such a way as to counteract that change to establish a new equilibrium
How does concentration of gases or aqueous substances affect equilibrium?
Increase: Equilibrium shifts to the opposite side of the reaction to remove the increased substance
Decrease: Equilibrium shifts to the same side of the reaction to produce more substance
How does pressure of reactants affect equilibrium?
Increase: Volume decreases. Equilibrium shifts to the side with fewer moles to reduce pressure
Decrease: Volume increases. Equilibrium shifts to the side with more moles to increase pressure
How does temperature affect equilibrium?
Increase: Favours endothermic reactions
Decrease: Favours exothermic reactions
*Exo. occurs at the opposite direction of end.
How does the presence of a catalyst affect equilibrium?
Quickens the attainment of equilibrium
Does not affect position of equilibrium
What is the equation for Haber Process?
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
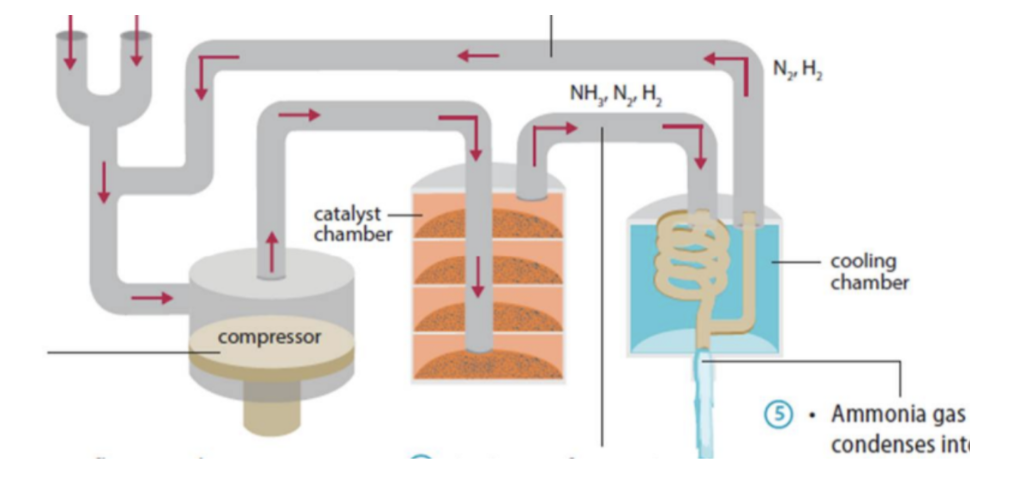
Describe the Haber process
N2 and H2 are mixed in the ratio of 1:3 b volume
The gas mixture is compressed
Compressed gases flow over the catalyst and are heated
A mixture of NH3, N2 and H2 is obtained and cooled
NH3 condenses into a liquid pumped into tanks and stored under pressure
Unreacted N2 and H2 are transferred back into the converter to be recycled
What are the conditions for the Haber process?
Pressure of 250atm
Temperature of 450ºc
How is hydrogen obtained for the Haber process?
From the cracking or breaking down of crude oil fractions
How is nitrogen obtained for the Haber process?
From the fractional distillation of liquid air
Why is an Fe catalyst used in the Haber process?
Speeds up the forward and reverse reactions, which are slow due to the compromise temperature and pressure
Cost of using catalyst is lower in the long run since it can be reused and regenerated
What is the effect of pressure in the Haber process?
Higher pressure → higher ammonia yield and faster reaction
*250atm compromised pressure as maintaining a high pressure is costly
What is the effect of temperature in the Haber process?
Lower temperature leads to higher yield of ammonia as it reduces the decomposition of ammonia
*450ºc is a compromised temperature as lower temperature leads to a slower reaction
How does temperature affect rate and yield of reactions in general?
Low temp: Low rate, high yield
High temp: High rate, low yield
Moderate temp: Moderate yield and rate
With high rate, frequency of effective collisions is lower