Chapter 5: Stoichiometry
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
Stoichiometry
the calculation of quantities of any substances involved in a chemical reaction from
the quantities of the other substances.
refers to the ratios of substances in a chem rxn and thus requires a balanced chemical equation
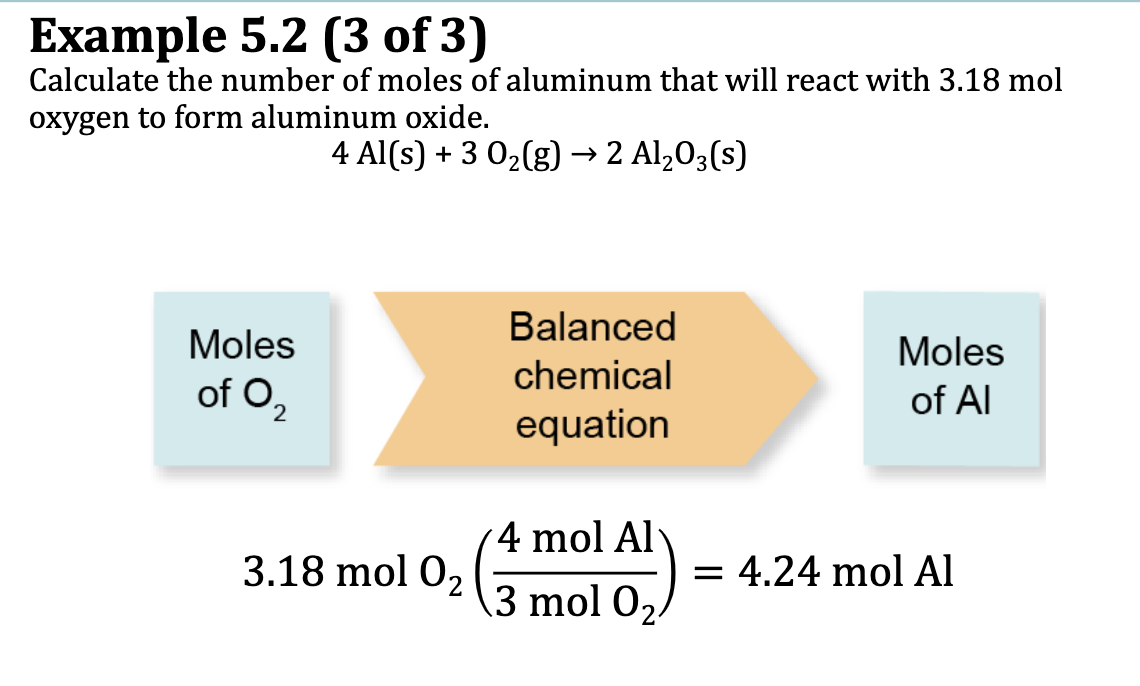
Ex. 5.2: Calculate the number of moles of aluminum that will react with 3.18 mol oxygen to form aluminum oxide
4Al (s) + 3 O2 (g) —> 2Al2O3 (s)
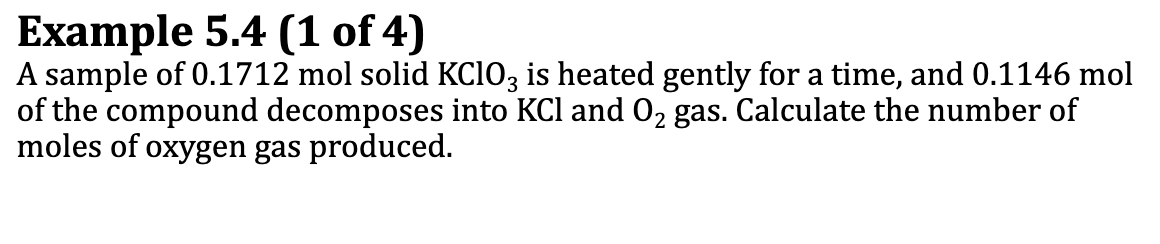
Ex. 5.4
First write the balanced equation for the reaction
2KClO3(s) → 3 O2 (g) + 2KCl (s)
Only 0.1146 mol KClO3 reacts
Conversion: Mol KClO3 -balanced chemical equation→ MolO2
0.1146 molKClO3 (3 mol O2/2 mol KClO3) = 0.1719 mol O2
Mass Calculations Use Molar Mass
Reacting ratios are mole ratios, not mass ratios
Use molar mass to convert masses to moles and vice versa
This Reaction can be Interpreted in 2 ways: 2 P (s) + 3 Cl2 (g) → 2 PCl3 (l)
2 atoms of phosphorous react with 3 molecules of Cl2 to produce 2 molecules of PCl3
Cl2 is a diatomic molecule, which is why we used “molecules”
2 moles of phosphorus react with 3 moles Cl2 to produce 2 moles of PCl3
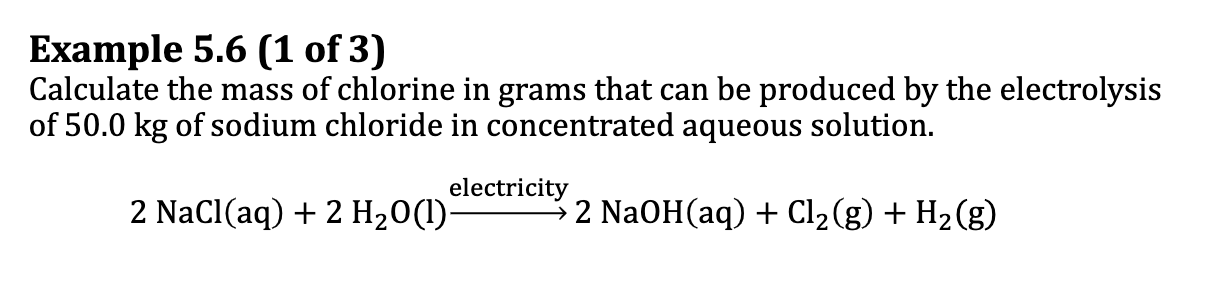
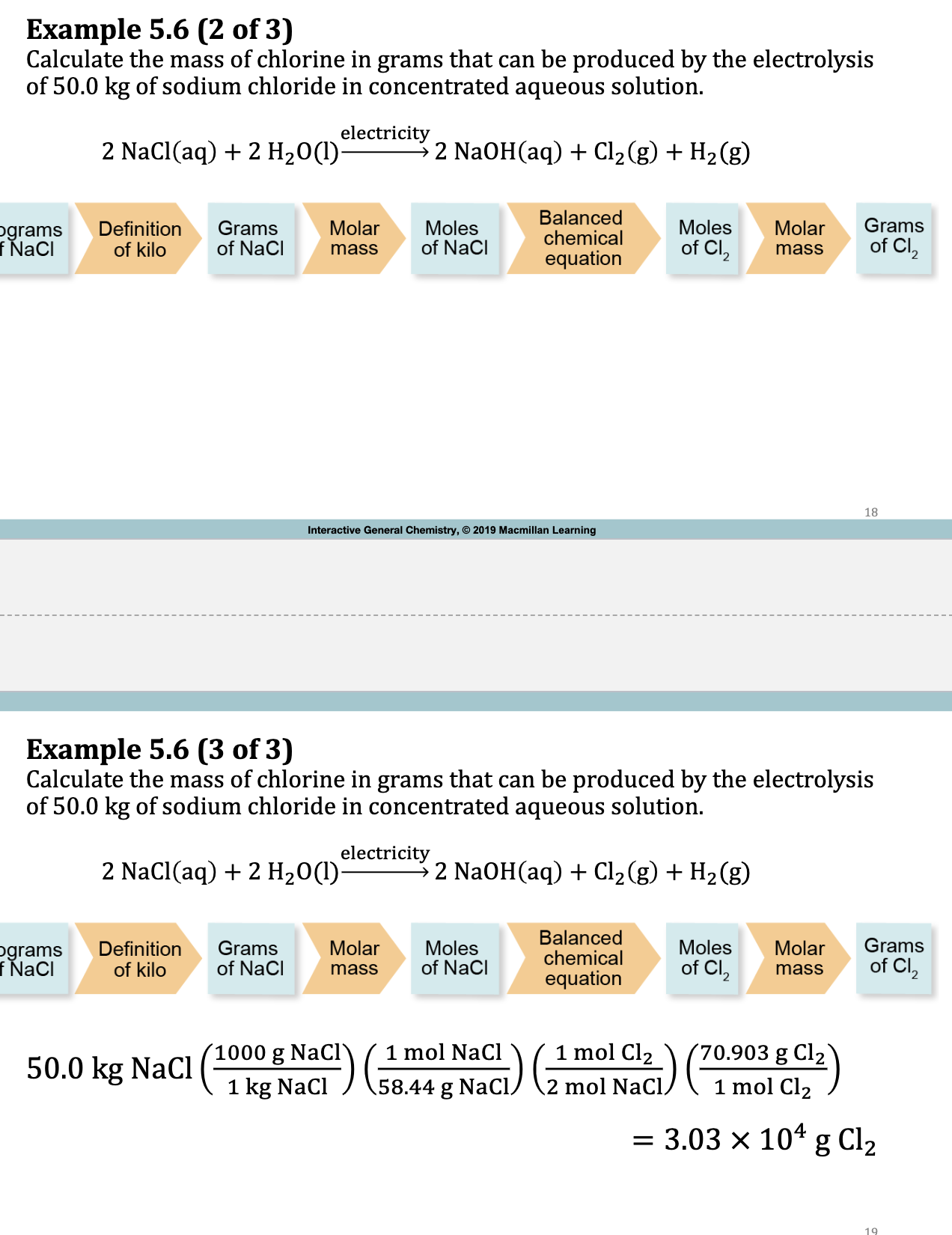
Ex. 5.6
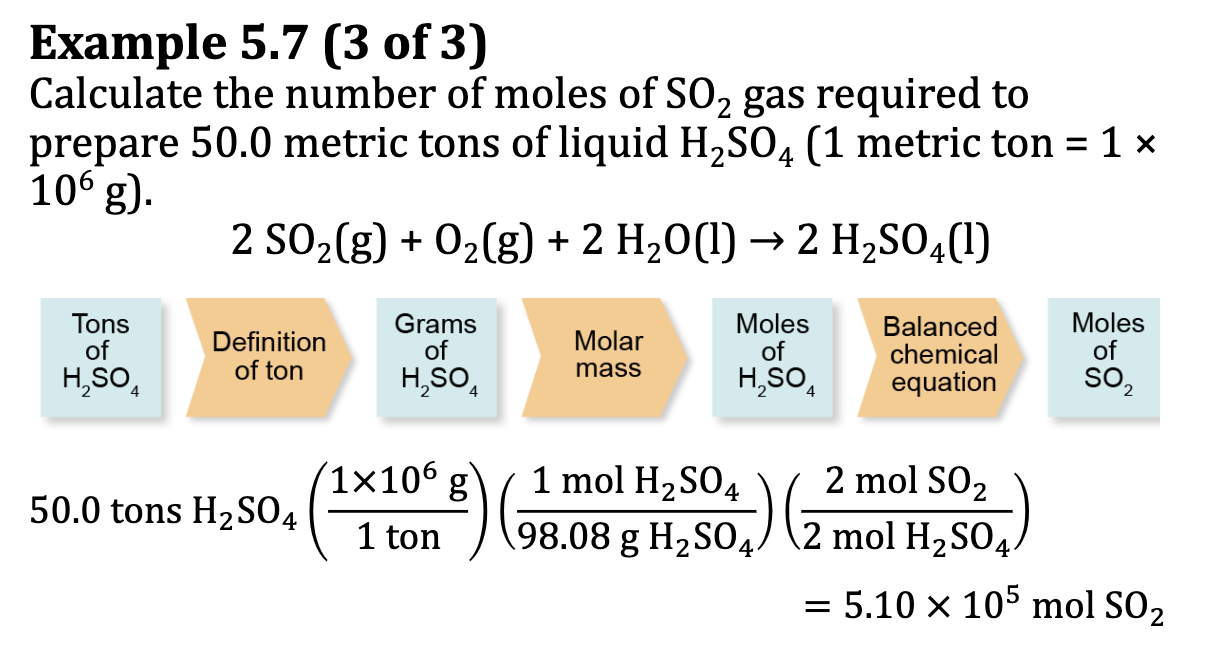
Ex. 5.7
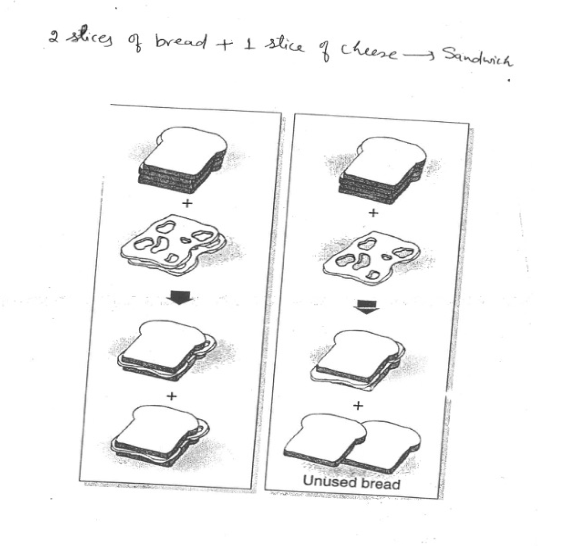
Limiting Reactant Concept
Thus far, all calculations have assumed that sufficient amounts of all reactants are present. This is not always the case
When 1 reactant is used up before the other; this reactant limits:
the amount of any other reactant that can react
how much product can be produced
cheese = limiting reactant; bread = excess reactant
Limiting Reactant Terminology
The reactant that is used up is the limiting reactant and is present in limiting quantity
Any reactant that is left over is present in excess
The reaction has gone to completion when the limiting reactant is used up

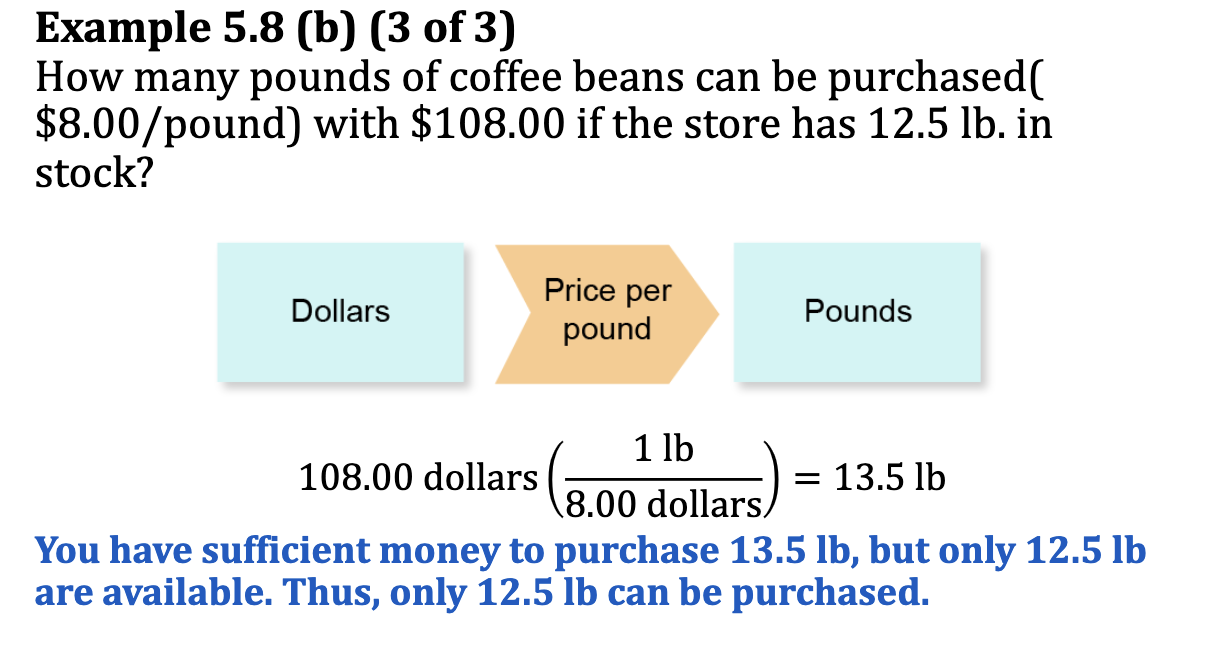
Ex. 5.8
Steps in Solving a Limiting Reactant Problem
Limiting reactant problems have the quantities of 2 or more reactants given
Identify the limiting reactant
Base all subsequent reacting-ratio calculations on the amount of limiting reactant
3 Ways to Identify a Limiting Reactant
• Separately calculate the amount of product that could form
from each reactant. The reactant that produces the smaller
amount of product is limiting.
• Divide the moles of each reactant by its coefficient. The
reactant with the smallest quotient is the limiting reactant.
• Find the amount of each reactant needed to completely use up
the other reactant. Disregard the scenario that requires more
of a reactant than is actually present.
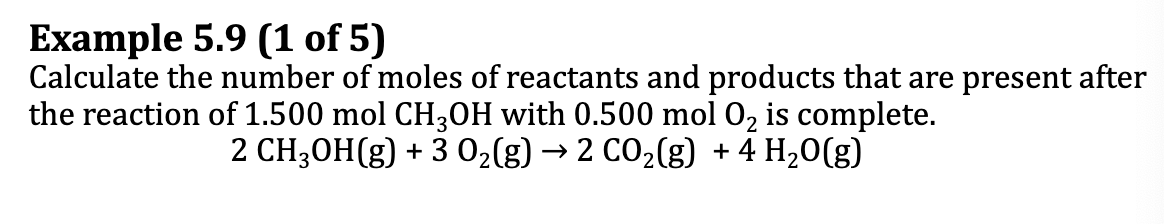
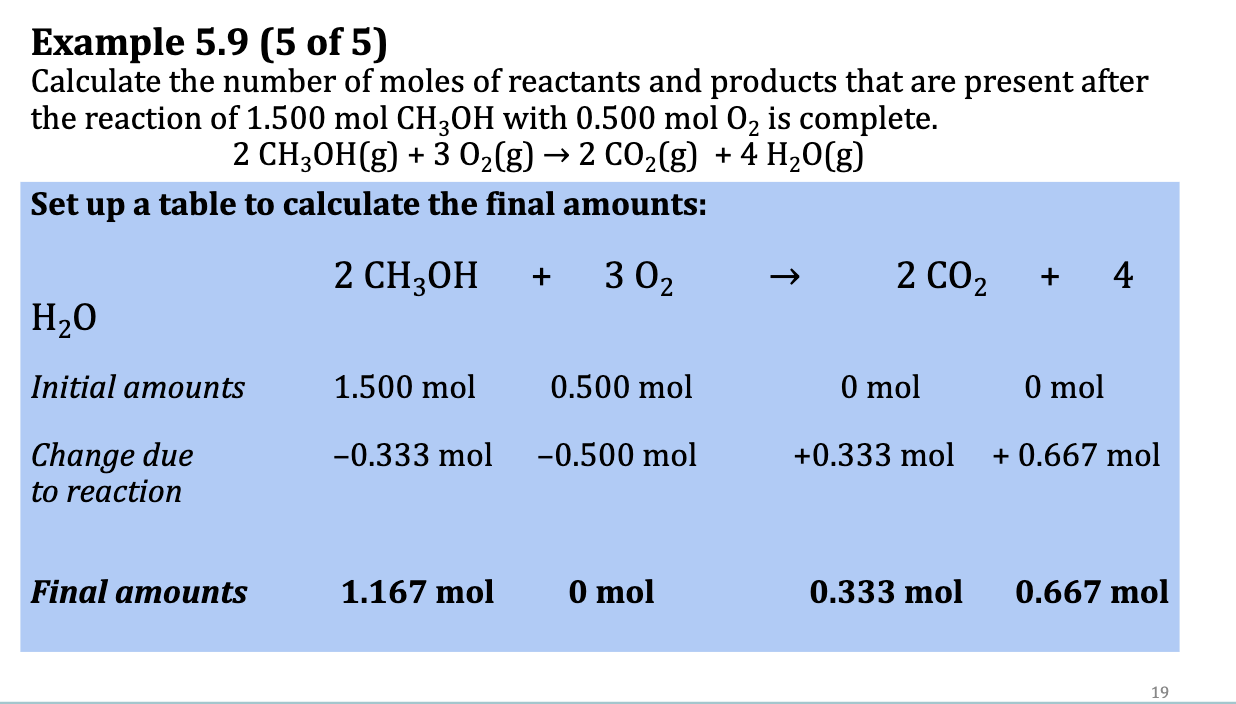
E.x. 5.9
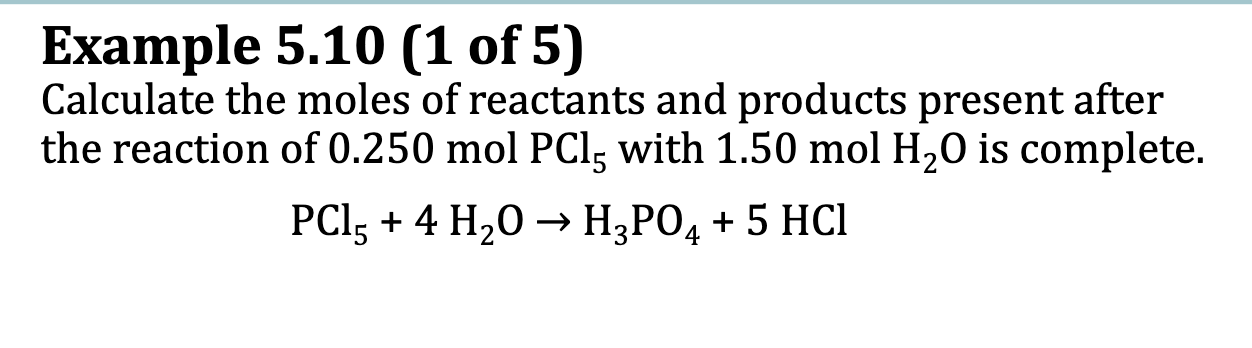
Ex. 5.10
How to find Limiting Reactant
moles divided by the # of coefficient

Ex. 5.12
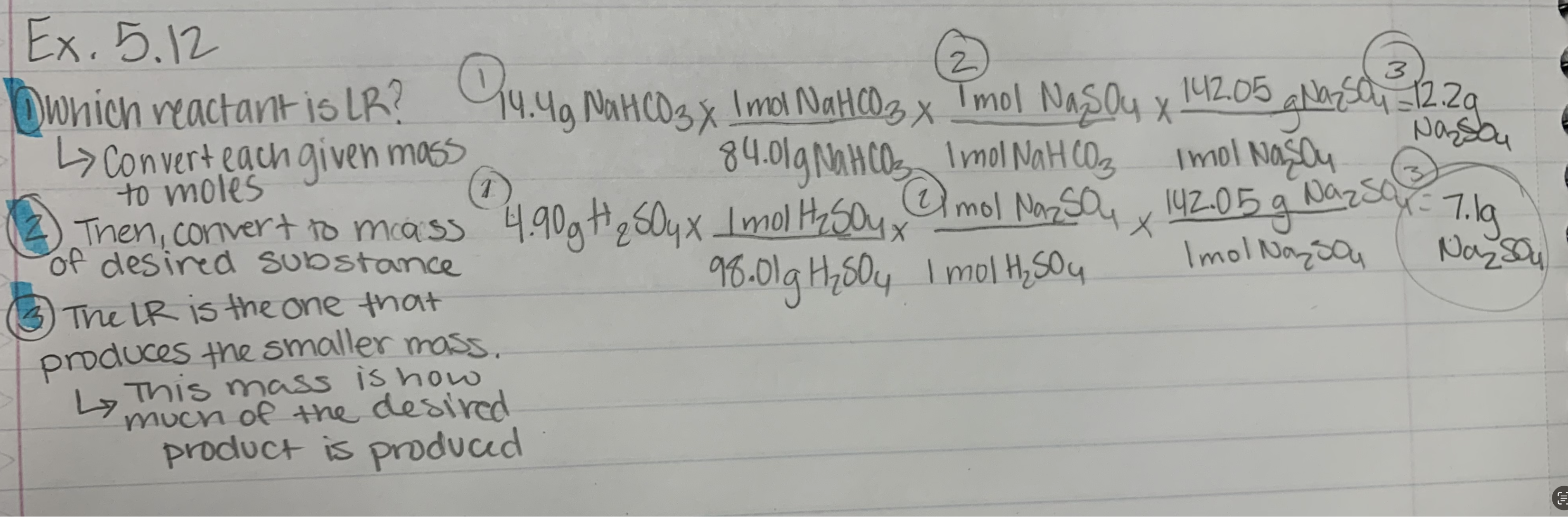
Theoretical Yield
the maximum amount of product that can be formed from a reaction, based on the amounts of reactants available (calculated using the amount of limiting reactant present)
basically what we have been doing
Actual Yield
amount of product that is actually obtained in an experiment
A.Y. = (Percent Yield)(Theoretical Yield)/100%
Percent Yield
defined as 100% times the ratio of the actual yield to the theoretical yield
P.Y. = (A.Y./T.Y) x 100%
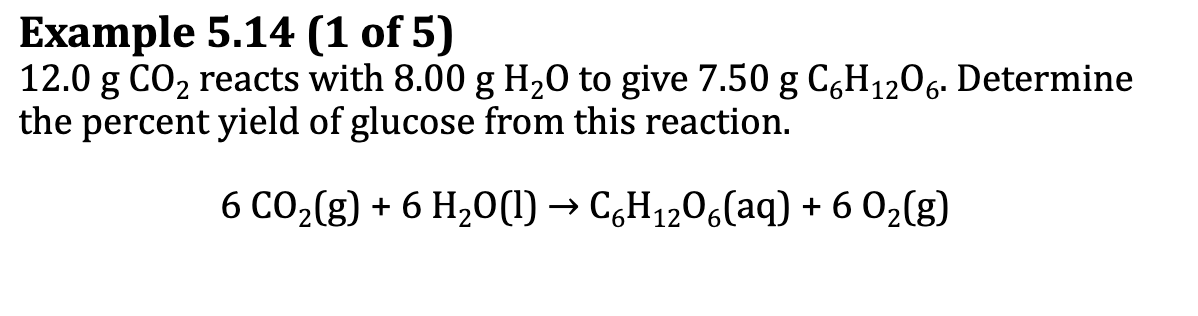
Ex. 5.14

What is a solution?
A homogeneous mixture of 2 or more substance
What is a solvent?
the component in a mixture that is present in the greatest amount
What is a solute?
In a solution, the substance(s) dissolved in the solvent
Concentration
expresses the quantity of a solute in a given quantity of solvent or solution
Solutions with a relatively large amount of solute per volume of solvent are ______
concentrated
Solutions with a relatively small amount of solute per volume of solvent are ______
dilute
Molarity, M
a type of concentration, defined as the number of moles of solute per liter of solution
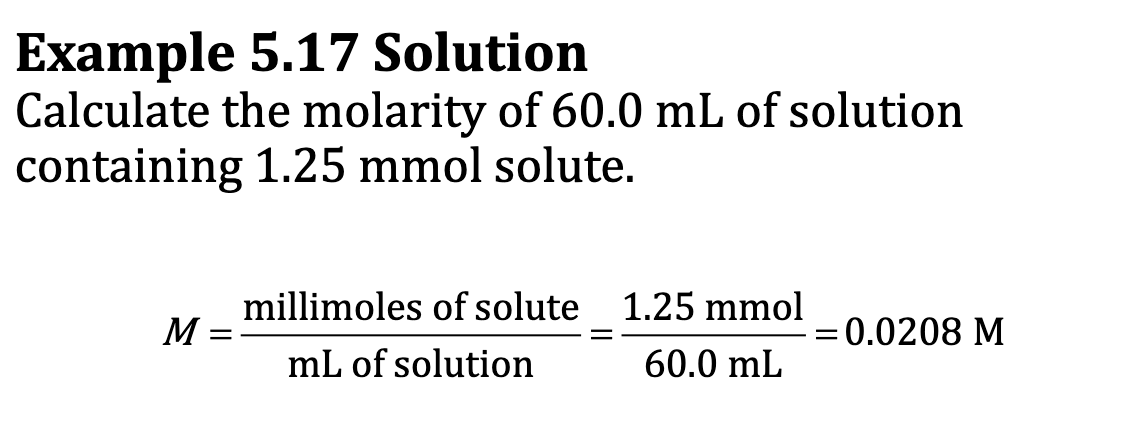
M = moles of solute/ liter of solution
.50 M NaCl means that there are .50 moles of NaCl in each 1.0 L of the solution
can also be millimoles of solute per milliliter of solution
M = moles of solute/liter solution = millimoles of solute/milliliter of solution
Ex. 5.16

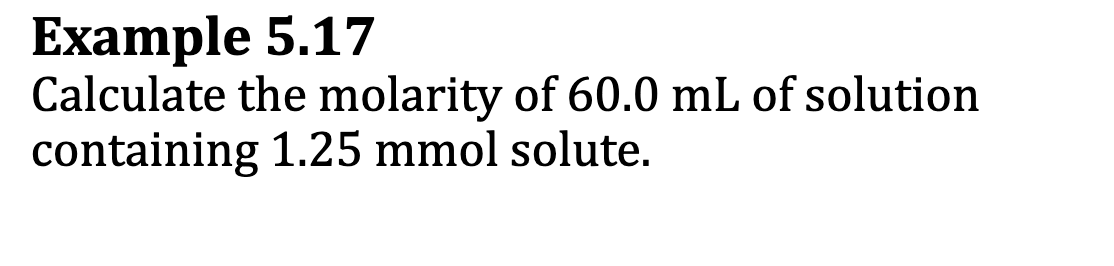
Ex. 5.17
1 mml = 1mmL

Ex. 5.18
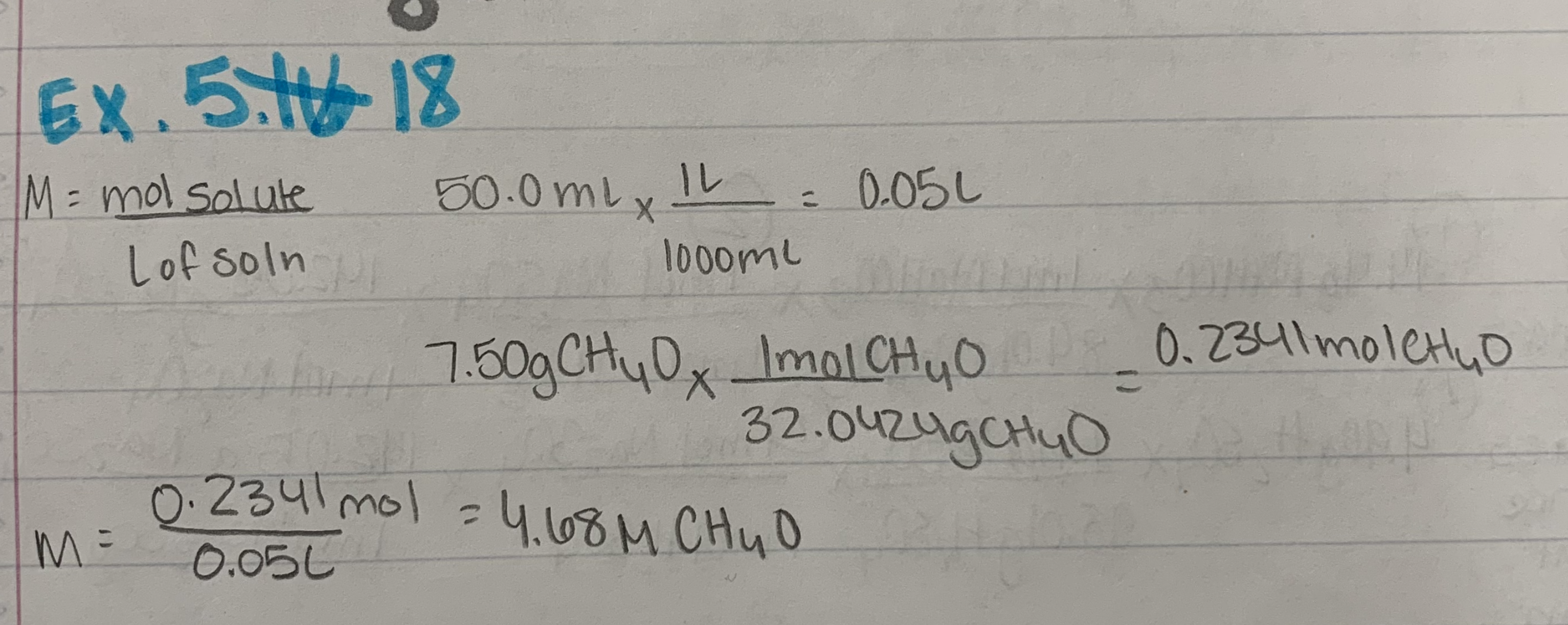
Molarity as a Conversion Factor (example): Determine the number of moles of solute in 3L of a 1.5 M soln
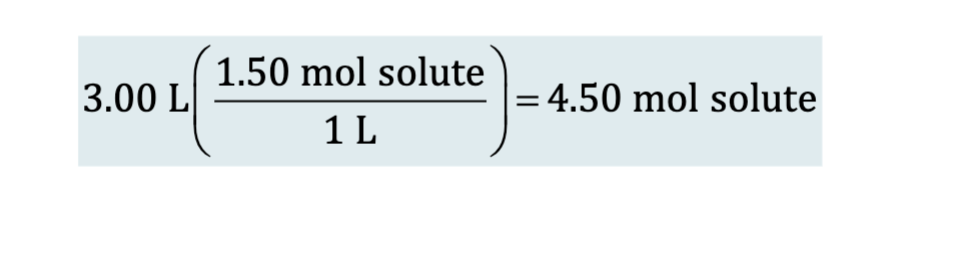
Dilution
Dilution is the process of adding more solvent to decrease the concentration of solute in a solution.Dilution is the process of adding more solvent to decrease the concentration of solute in a solution.
Dilution Equation
When a soln is diluted with solvent the number of moles of solute does not change
Before the dilution, M1 x V1 = mol of solute
After the dilution, M2 x V2 = mol of solute
Because the number of moles of solute has not changed, these 2 expressions are equal to one another
Example: Calculate the concentration of soln when .750L of 1.60M NaCl is diluted with water to make 3L of soln?
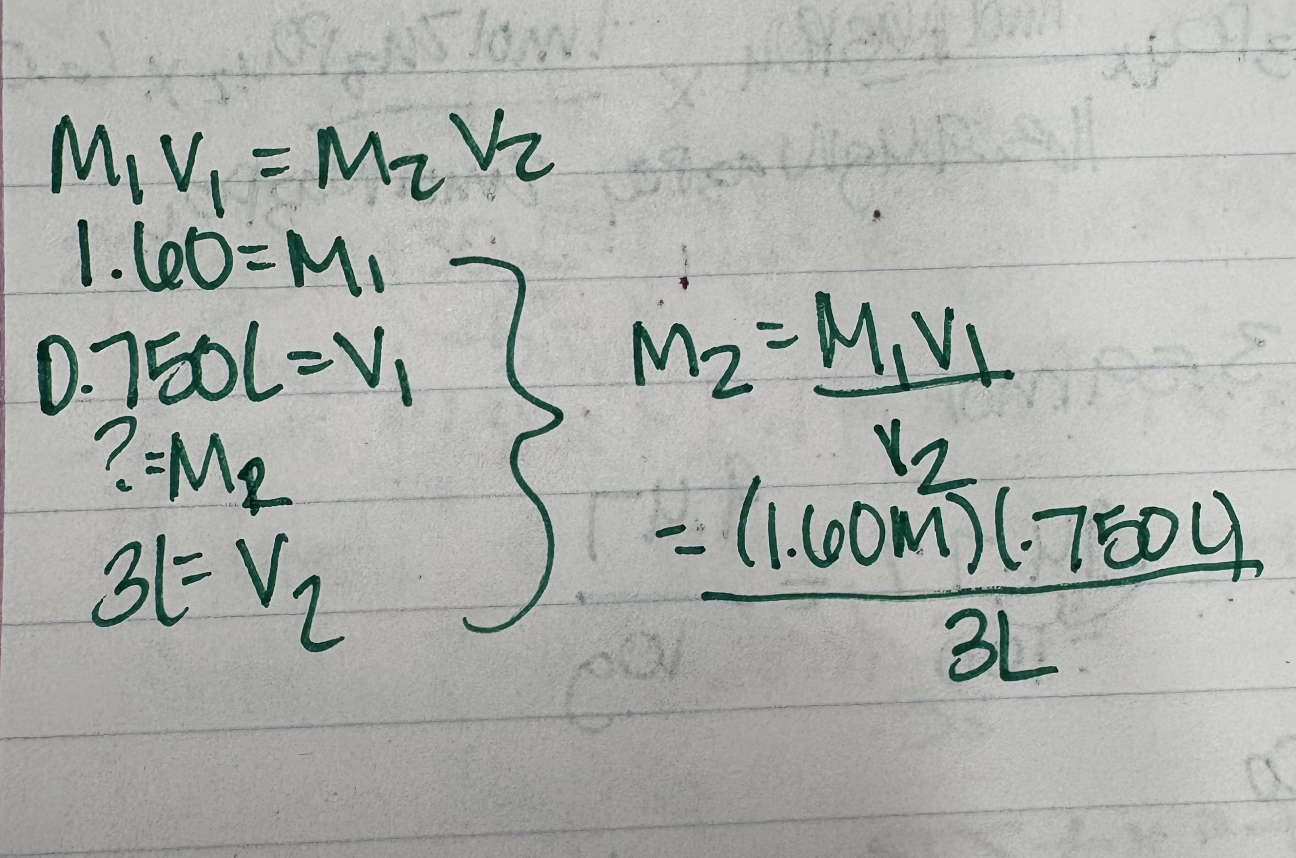

Ex. 5.21

Mixing Solutions of Unequal Concentration
E.x. 5.22:
Calculate the final conentration after 1.25L of 2.25 M NaCl is added to 3.50L of 2.45M NaCl
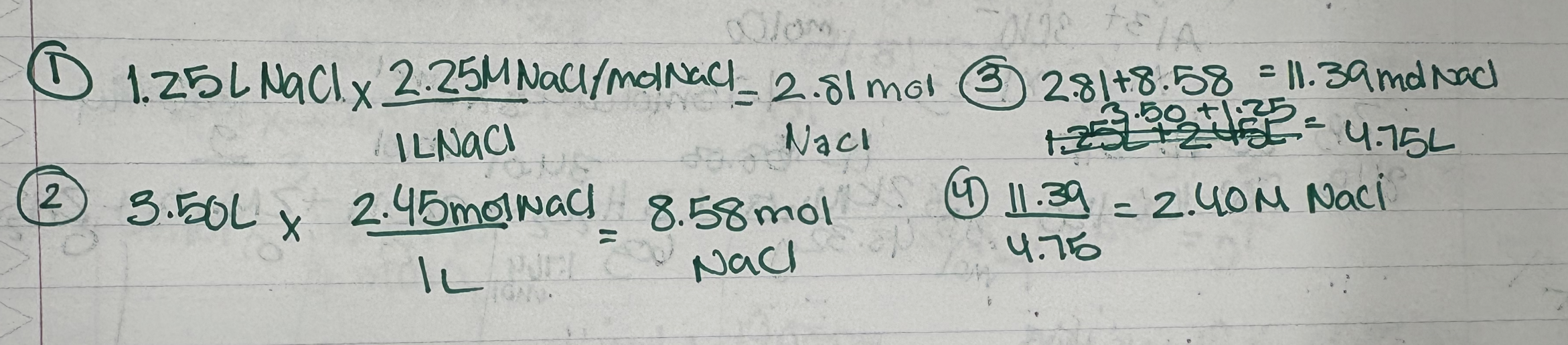
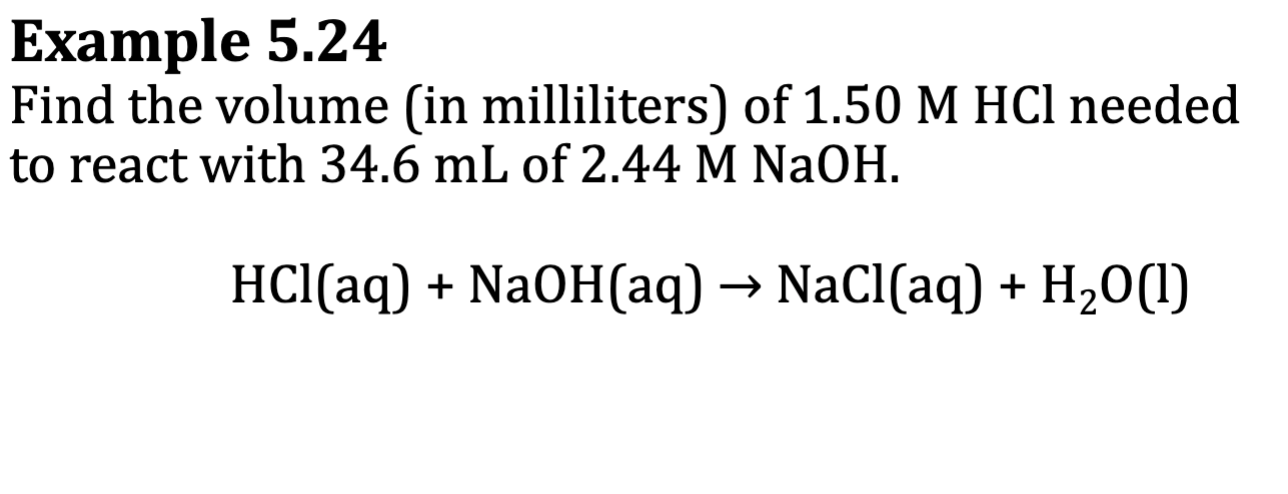
E.x.: 5.24
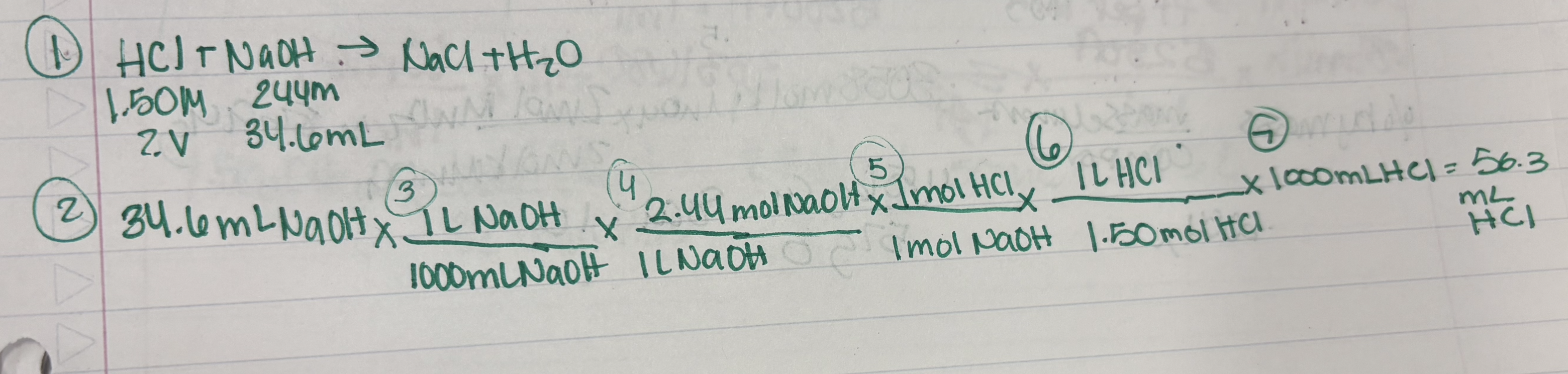
Strong electrolytes _______ into _____ when dissolved in water
dissociate; ions
The ______ of any ion is the number of moles of that ion per liter of solution
molarity
In a 1.0M soln of AlCl3(aq) the ion concentrations are ___ Al³+ and ___ Cl^-
1.0M;3.0M
If the quantity of one element is given, it is not a __________ equation. If for example, O2 is not given an amount, that means there is an ____ supply.
limiting reactant; infinite
Example: Calculate the number of moles of solid mercury (I) oxide, Hg2O, that can be produced by the combo rxn of excess oxygen gas with 25 mL of liquid mercury (density = 13.53 g/mL). Also, calculate the number of molecules of oxygen required
then multiply by Avogadros number oops
Ex. 5.30: Determine the number of SO2 molecules that are required to. combine with excess O2 to produce 0.751 molSO3:
2SO2 (g) + O2 (g) → 2 SO3 (g)
Example: Determine the mass of Ba(NO3)2 that is required to provide 0.750 mol Ba²+
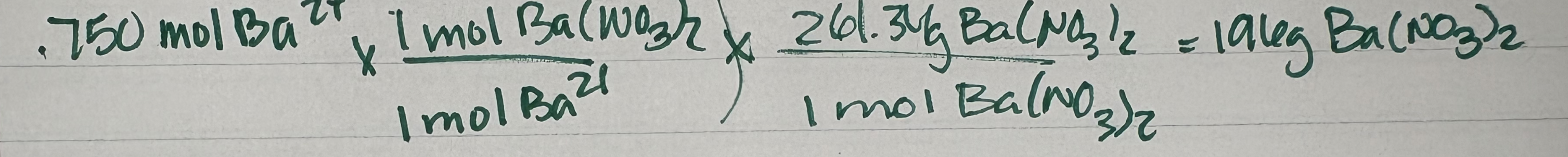

227 mol H3PO4× 1 mol (NH4)3PO4/1molH3PO4×3mol N/1mol (NH4)3PO4 = 681mol N
Titration
Titration is a laboratory technique for determining the number of moles of a substance dissolved in an aqueous solution (sample
solution).
• One reactant, the standard solution, has a precisely known concentration.
• The volumes of the standard solution and the sample are both carefully measured.
• The mole ratios and volumes are used to calculate the sample concentration.
Ex. 5.32: Calculate the concentration of an HCl solution if 25.00 mL HCl is titrated with 2.000 M NaOH. The initial buret reading is 2.17 mL, and the final buret reading is 39.42 mL.
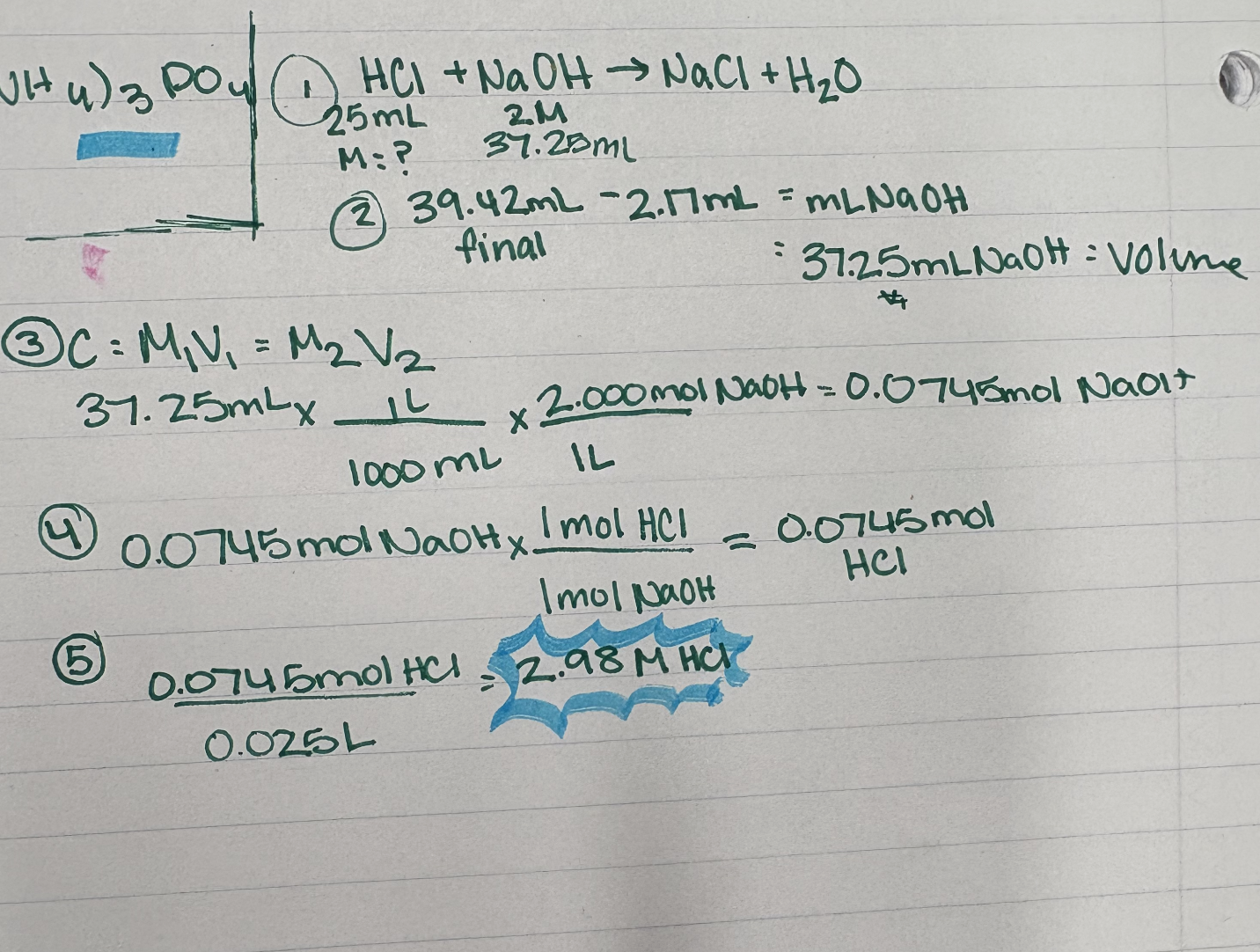
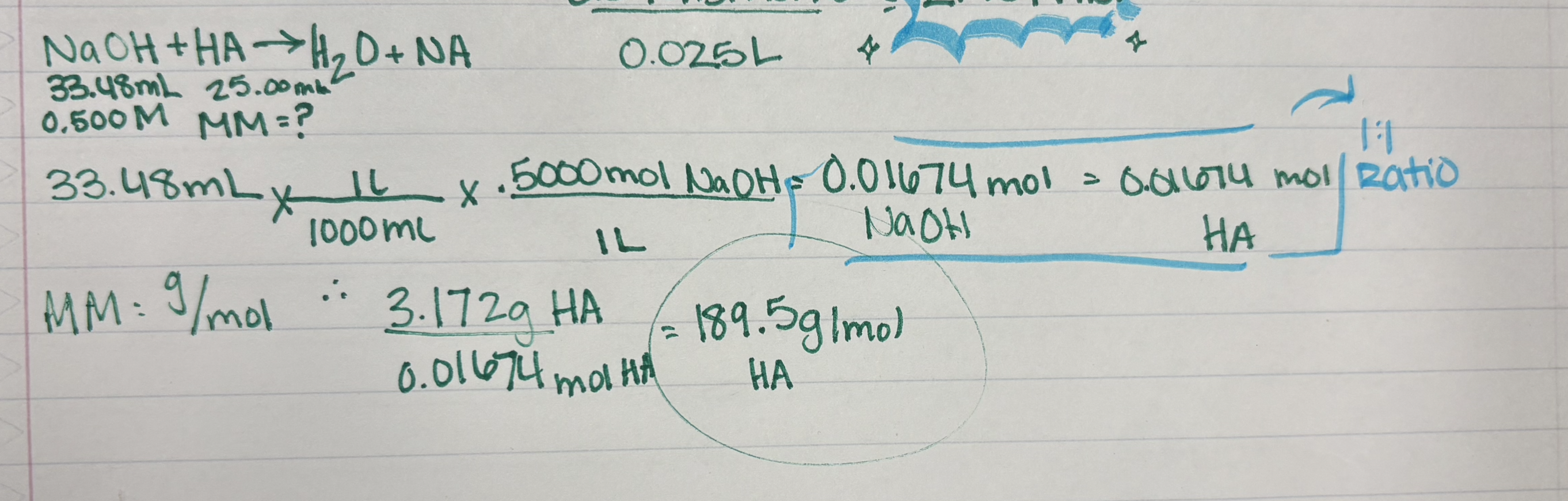
Example: Calculate the molar mass of a monoprotic acid (HA) if it takes 33.48 mL
of 0.5000 M NaOH to neutralize 25.00 mL of a solution of the unknown
acid containing 3.172 g of the acid.
HA(aq) + NaOH(aq) → NaA(aq) + H2O(l)