PMI 128: Lecture 11-13
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
72 Terms
What are “Anti-viral defenses”?
The various mechanisms, both innate and acquired, that the body uses to combat viral infections
What types of mechanisms are involved in Intrinsic Anti-viral defenses?
Physical, chemical, and physiologic defenses
Immune defenses require ____ of “non-self” pathogen.
Recognition
What is Innate immunity?
Preexisting or rapidly inducible host effector systems
What does Innate immunity do in anti-viral defense?
Provides time for development of specific antibody and T cell responses
What is Adaptive Immunity?
Inducible host effector systems targeted ro the specific viral agent
What does Adaptive Immunity do in anti-viral defense?
Clears viral infection, limits repeated infections with same virus
Why is recognition of “non-self” important for immune antiviral responses?
Recognition of “non-self” allows immune cells to distinguish pathogens from the body’s own cells, enabling a targeted and effective response without damaging host tissues
What is a key characteristic of non-immune antiviral defenses like physical, chemical, and physiologic defences?
They do not rely on recognition of a “non-self” pathogen, instead they act broadly and immediately to prevent viral entry or spread
What is apoptosis?
Cell-suicide
Form of programmed cell death
How does apoptosis help in antiviral defense?
When a cell is infected by a virus, it can undergo apoptosis to limit viral replication and spread.
Is a method of antiviral defense
What are the pathways of Apoptosis?
Intrinsic
Extrinsic
What triggers the Intrinsic pathway of Apoptosis?
Cellular stress
DNA damage, Oxidative stress, Viral infection, Growth factor deprivation, Oncogene activation
State the order of the Intrinsic pathway of Apoptosis
Cellular stress→Pro-apoptotic BCL2 protein activation via BH3-only proteins→Cytochrome-c release→Apoptosome formation (APAF1, procaspase-9, dATP)→ Activation of executioner caspases
What triggers the Extrinsic pathway of Apoptosis?
Death receptors on the cell surface
TNFR, CD95/FAS
State the order of the Extrinsic pathway of Apoptosis
Death receptors→ Death-receptor ligation→ Adaptor recruitment→ Procaspase-8 recruitment→ Caspase-8 activation→ activation of executioner caspases
What determines whether an adaptive response is triggered?
Pattern recognition receptors (PRRs)
What happens when a pathogen is recognized by the innate immune system?
Activation of dendritic cells and NK cells, cytokines and complement release, and triggering of the adaptive immune system
What happens if a pathogen is not recognized in the innate immune system?
No adaptive immune response
What are PRRs and what do they recognize?
Pattern recognition receptors, they recognize pathogens-specific molecules, aberrant localization of foreign or self molecules, or abnormal molecular complexes
What can PRR activation lead to besides infection resolution?
Inflammation diseases or autoimmunity
What are Humoral Factors?
Soluble molecules that circulate in the bloodstream and lymph, acting as intermediaries in the immune response
What Humoral factors did we go through in lecture?
Natural antibodies
Complement proteins
What are Natural Anitibodies (NAbs)?
Immunoglobins present in the blood of healthy individuals before exposure to an antigen or immunizations
What are Complement proteins?
Group of serum proteins involved in the control of inflammation, the activation of phagocytes, and the lyric attack on cell (or viral) membranes.
These colecules are produced by the liver
What are some pattern recognition receptors that we went over in lecture?
C-Type lectin receptors (CLRs)
Toll-like receptors (TLRs)
RIG-I-receptors (RLRs)
Nucleotide-binding oligomerization domain-like receptors (NLRs)
What are CLRs?
c-type lectin receptors
Transmembrane proteins localized at the plasma membrane
recognize glycine’s from the wall of fungi and some bacteria
What are TLRs?
Toll-like receptors
Transmembrane proteins localized either at the plasma membrane or in endosomes
Broad range of specificities recognized proteins, nucleic acids, and glycans
What are RLRs?
RIG-I-Like receptors
Cytoplasmic sensors of viral RNA
Signal via mitochondrial adapter proteins MAVS
Trigger antiviral responses including the production of type 1 interferon
What are NLRs?
Nucleotide-binding Oligomerization domain-like receptors
Cytoplasmic sensors
Multiple subfamilies: NLPRs recognize bacterial, viral, parasitic, and fungal PAMPs AIM2 detects viral and bacterial DNA
Form multiprotein signaling complexes known as inflammasomes
What transcription factors are activated by TLRs in response to viral PAMPs?
NF-kB
IRP3
IRF7
TLRs signal through ________
TIR domain-containing adaptors
e.g. MyD88, TRIF
What adaptor is used by most TLRs to induce inflammatory cytokines?
MyD88
Which adaptor does TLR3 use to produce type 1 interferons?
TRIF
What is SARM and what does it do?
An adaptor that inhibits TRIF-dependent signaling
What the two main pathways downstream of TLR4?
MyD88 and TRIF
What does it mean when PRR signaling is “divergent”?
One receptor recruits multiple adaptors, leading to distinct cellular outcomes depending on adaptor use or cell types
What does it mean when PRR signaling is “convergent”?
Multiple receptors use the same adaptor, leading to a similar cellular response
What kind of immune response does the RIF-I and MDA5 signal pathway trigger?
Antiviral innate immune response
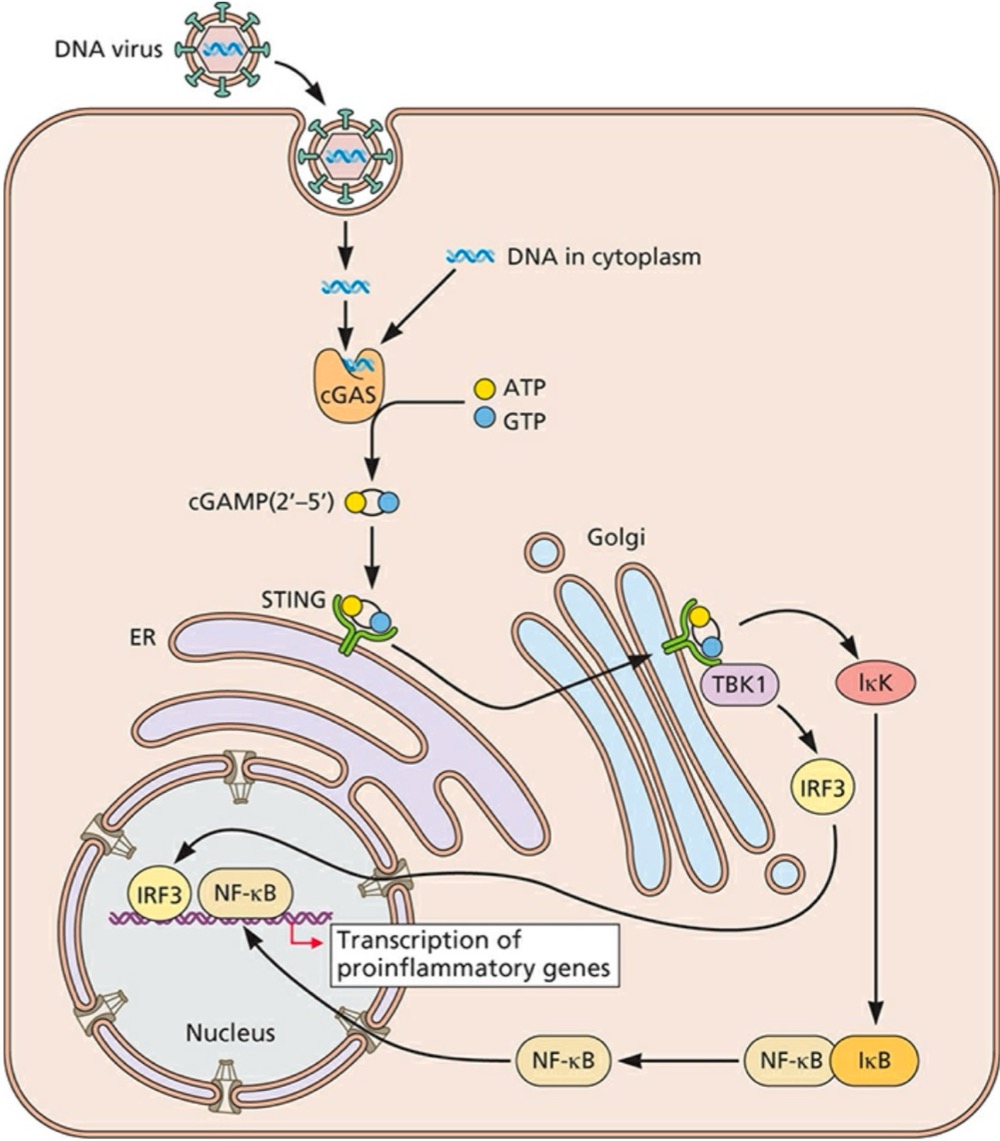
Double-stranded DNA in the cytoplasm is detected by what?
Cyclic GMP-AMP (cGAMP) synthase (cGAS), which synthesizes cGAMP (2’-5’) as its second messenger molecule
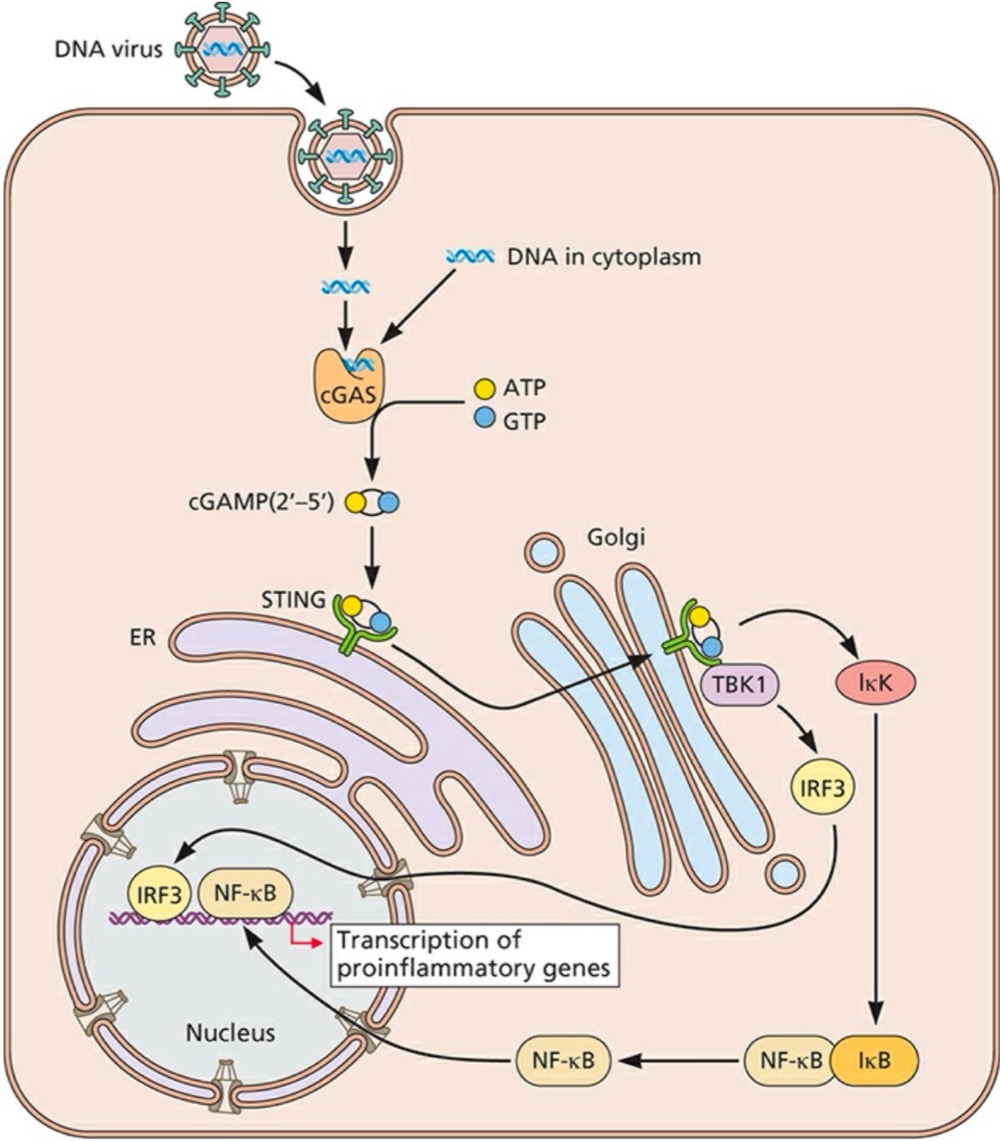
What does cGAMP (2’-5’) do after its synthesized?
Binds and activates the endoplasmic reticulum (ER)-resident receptor STING (stimulator of interferon genes)
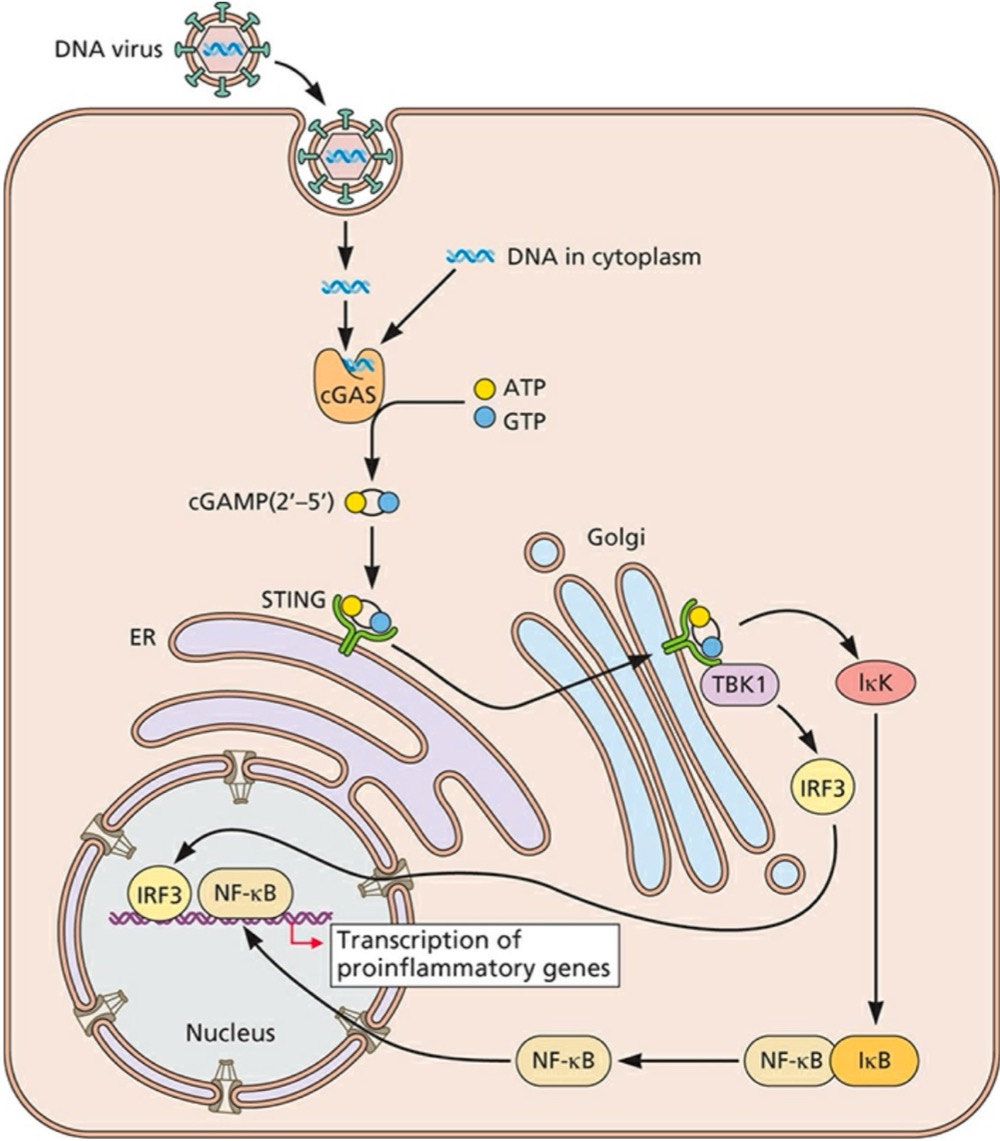
What can STING do once its activated?
Translocated to a perinuclear Gogli compartment, where it binds to TBK1 (TANK-binding kinase 1) to activate IRF3 and induce NF-kB activation
What are the systemic effects of inflammatory cytokines?
Feaver, Fatigue, lethargy
Hematopoiesis, Mobilization of lymphocytes
What does type 1 interferon synthesis and paracrine signaling result to?
Synthesis of interferon stimulated genes
What are the Type 1 Interferon receptors shown in lecture?
IFN-αs and IFN-β
What are the Type 2 Interferon receptors shown in lecture?
IFN-Υ
What are the Type 3 Interferon receptors shown in lecture?
IFN-λs
How is IFN-λ produced?
By intestinal epithelial cells (IECs) and signals via the IFN-λ receptor (IFNλR) on these cells
Dendritic cells provide ______ to naive T cells
Cytokine signals and peptide antigens
What can NK cells help distinguish?
Normal healthy cells from infected cells missing self receptors
What is the purpose of virus-encoded mechanisms that modulate NK-cell activity?
To evade immune detection and killing by natural killer (NK) cells by interfering with NK-cell recognition or activation pathways
What is Strategy 1 used by viruses to evade NK cells?
Inhibition by a viral protein with homology cellular MHC class 1 proteins
What is Strategy 2 used by viruses to evade NK cells?
Inhibition of production or cell surface localization of human MHC class 1, resulting in an increase in the quality of host HLA-E (or HLA-C) on the target surface
What is Strategy 3 used by viruses to evade NK cells?
Release of virus-encoded cytokine-binding orteins that block the action of NK-cell-activating cytokines
What is Strategy 4 used by viruses to evade NK cells?
Inhibition of action of NK-cell-stimulating cytokines by binding these cytokines or by producing a chemokine antagonist
What is Strategy 5 used by viruses to evade NK cells?
Effect of newly produced virus particles, which can engage the NK cell, block an inhibitory NK-cell receptor, or infect the NK cell itself to disrupt various effector functions or even kill the cell.
What natural antibodies did we go over in lecture?
IgM, IgG or IgA
Poly-specific low-affinity antibodies
They also react with proteins, lipids or carbohydrates
What is Opsonization?
Enhanced phagocytosis by coating of the viral surface with C’ (complement receptor) or antibody (Fc receptor)
What is Chemotaxis?
Process whereby chemicals (C’) direct cell movement and orientation
How can the complement system be activated?
By three pathways: classical, lectin, and alternative
What does the Classical pathway in the complement system use?
Antibody complex
What does the Lectin pathway in the complement system use?
MBL-carbohydrate
What does the Alternative pathway in the complement system use?
C3b-microbe
What does the Complement system enhance and activate?
Enhances chemotaxis and phagocytes
Activates immune cells
What does Factor H do?
Part of the regulation of the complement cascade
Inhibits factor B binding to C3b
Accelerates the decay of C3bBb
Cofactor for factor 1 in cleaving C3b to iC3b
Inhibits phagocytosis and kills by MAC
What are Macrophages?
Long-lived phagocytic cells involved blood and tissues derived from bone marrow
Engulf, internalize and destroy viruses
Present antigens to T cells to elicit adaptive anti-viral immunity
How are macrophages formed?
Begin as stem cell in bone marrow, stay 10-20 hrs in circulation, leave blood to tissues and transformed into large macrophage cells, and life span is up to few months in tissues
What are the different types of macrophages?
Kupffer, Microglia, Reticular, tissue history ties, alveolar cells
How do the different types of macrophages differ?
Organs in which they reside
Phagocytosis is part of the _______
Innate immune system
What is the primary role of innate immunity?
To limit the viral infection
What can adaptive immunity do?
Prevent, limit, and reduce infection
Clear viral infections