Biochemistry Chapter 17
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
88 Terms
what does the citric acid cycle harvest
high energy electrons
citric acid cycle
series of oxidation– reduction reactions that result in the oxidation of an acetyl group to two molecules of CO2 – the final pathway for the oxidation of fuel molecules – oxidation generates high-energy electrons used to power ATP synthesis – important sources of precursors for biosynthesis – also called the tricarboxylic acid (TCA) cycle or Krebs cycle
CAC
citric acid cycle
what is the final pathway for the oxidation of fuel molecules
CAC
what reactions make up the CAC
oxidation-reduction
acetyl CoA
acetyl coenzyme A. most fuel molecules enter the CAC as this
what do most fuel molecules enter the CAC as
acetyl CoA
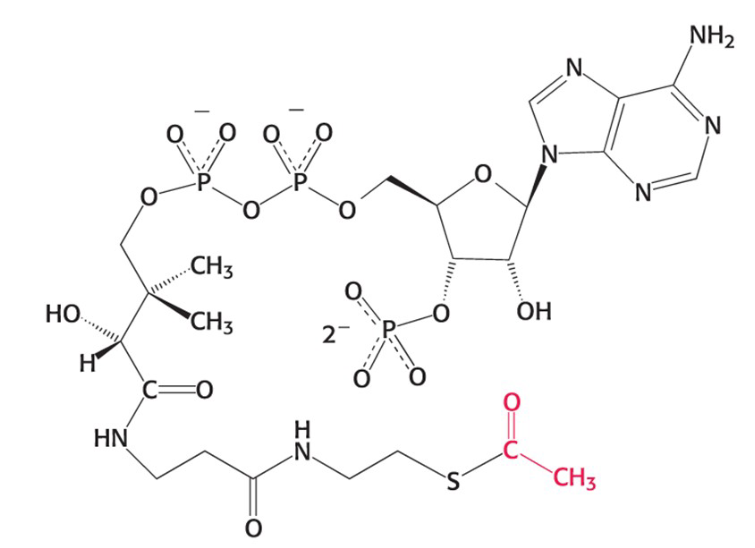
acetyl CoA
pyruvate dehydrogenase complex
a large enzyme complex that oxidatively decarboxylates pyruvate to acetyl CoA under aerobic conditions
where do reactions of the pyruvate dehydrogenase complex and CAC take place
in the mitochondrial matrix
what happens to acetyl CoA in the CAC (carbon)
two remaining carbons are completely oxidized to CO2
what are the two mitochondrial membranes
inner and outer
where is the matrix of the mitochondria
inside the inner mitochondrial membrane (all the way in center)
what does the CAC do
removes electrons from acetyl CoA and uses them to reduce NAD+ and FAD to NADH and FADH2
ETC
electron transport chain
electron transport chain
a series of membrane proteins that electrons released in the reoxidation of NADH and FADH2 flow through to generate a proton gradient across the inner mitochondrial membrane
why do protons flow through ATP synthase
to generate ATP from ADP and inorganic phosphate
what links glycolysis to the citric acid cycle
the pyruvate dehydrogenase complex
pyruvate dehydrogenase complex
a highly integrated unit of three distinct enzymes in the mitochondrial matrix. – oxidatively decarboxylates pyruvate to acetyl CoA
why does pyruvate dehydrogenase complex connect glycolysis to CAC
because the reaction catalyzed by it is an irreversible link
why must conversion of pyruvate to acetyl CoA steps be coupled
because free energy from decarboxylation step drives formation of NADH and acetyl CoA
what are the catalytic cofactors of conversion of pyruvate to acetyl CoA
thiamine pyrophosphate, lipoic acid, FAD
TPP
thiamine pyrophosphate
what are the stochiometric cofactors of conversion of pyruvate to acetyl CoA
CoA and NAD+
stochiometric cofactor
cofactor that functions as substrate
what does the citric acid cycle oxidize
two-carbon units
what does citrate synthase catalyze
the addition of acetyl CoA and oxaloacetate, yielding citrate and CoA
what is the reaction of citrate synthesis
aldol addition and a hydrolysis
what does citrate synthase proceed through
energy rich citryl CoA
how does the mechanism of citrate synthesis prevent undesirable reactions
minimizes hydrolysis of acetyl CoA to acetate and CoA side reaction, and exhibits sequential, ordered kinetics
what are the sequential, ordered kinetics of citrate synthase
Oxaloacetate binds first, followed by acetyl CoA. – Oxaloacetate induces a structural rearrangement that creates an acetyl CoA-binding site
what is citrate isomerized to
isocitrate
iron-sulfur protein (nonheme iron protein)
protein that contains iron that is not bonded to heme (ex: aconitase)
what does aconitase catalyze
the isomerization of citrate into isocitrate through dehydration step and hydration step
what is isocitrate oxidized and decarboxylated to
alpha ketoglutarate
what does isocitrate dehydrogenase catalyze
the oxidative decarboxylation of isocitrate, forming alpha ketoglutarate and NADH
NADH
high transfer-potential electron carrier
what does isocitrate dehydrogenase proceed through
the unstable oxalosuccinate
what is released from oxalosuccinate to yield alpha ketoglutarate
CO2
what is succinyl coenzyme A formed by
the oxidative decarboxylation of alpha ketoglutarate
what does the alpha ketoglutarate dehydrogenase complex catalyze
the oxidative decarboxylation of alpha ketoglutarate to succinyl CoA, yielding NADH
what regenerates NADH (CAC)
alpha ketoglutarate’s oxidative decarboxylation (alpha ketoglutarate + NAD+ + CoA)
what does succinyl CoA synthetase catalyze
the cleavage of a thioester linkage of succinyl Coa, yielding succinate
what is succinyl CoA synthetase coupled to and why
the phosphorylation of ADP or GDP because the delta G for the hydrolysis is comparable to that of ATP
is succinyl CoA synthetase catalyzation reaction reversible or irreversible
readily reversible
what may be coupled to the formation of succinate
ATP or GTP formation
what are the two isozymic forms of the enzyme succinyl CoA synthetase
GDP and ADP requiring
GDP requiring enzyme (succinyl CoA synthetase)
predominates in tissues performing anabolic reactions (ex: liver) and the GTP is used to power succinyl CoA synthesis
ADP requiring enzyme (succinyl CoA synthetase)
predominates in tissues performing large amounts of cellular respiration (ex: skeletal and heart mucle)
how is oxaloacetate regenerated
by the oxidation of succinate
what do succinate dehydrogenase, fumarase, and malate dehydrogenase catalyze
successive reactions of four-carbon compounds to regenerate oxaloacetate
what is generated in oxidation of succinate
FADH2 and NADH
why is it important for oxaloacetate to be regenerated
can initiate another cycle afterwards
where is FADH2 generated in CAC
succinate to fumarate
where is NADH generated in CAC
malate to oxaloacetate
succinate dehydrogenase
iron-sulfur protein. has isoalloxazine ring of FAD covalently attached to His side chain. is embedded in inner mitochondrial membrane. is directly associated with ETC
how is succinate oxidized to fumarate (by what)
by succinate dehydrogenase
what is the hydrogen acceptor in succinate to fumarate oxidation
FAD, because the free energy change is insufficient to reduce NAD+
how is fumarate hydrated to L-malate
by fumarase
fumarase
catalyzes the stereospecific trans addition of H+ and OH-, yielding only the L-isomer of malate
what is malate oxidized to
oxaloacetate
malate dehydrogenase
catalyzes the oxidation of malate, yielding oxaloacetate and NADH
what is the delta G of malate oxidation to oxaloacetate
significantly positive
what is the reaction of malate oxidation to oxaloacetate driven by
the use of the products - oxaloacetate by citrate synthase and NADH by the ETC
what is the net reaction of the citric acid cycle
Acetyl Coa + 3 NAD+ + FAD + ADP + Pi + 2H2O —> 2 CO2 + 3 NADH + FADH2 + ATP + 2H+ + CoA
what is important to know about the carbons in CAC
the two carbon atoms that enter each cycle as acetyl CoA are not the ones that leave as Co2 during the initial two decarboxylation reactions
what is the stoichiometry of the CAC (carbon, hydrogen, phosphoryl compound, water)
two carbon atoms enter in form of acetyl CoA, and two leave in the form of CO2. four pairs of hydrogen atoms leave in four oxidation reactions (yielding three NADH and one FADH2). one compound with high phosphoryl-transfer potential (usually ATP) is generated. two water molecules are consumed
how many reactions make up the full citric acid cycle
8 enzyme catalyzed reactions
what allows for substrate channeling in the CAC
a physical association of the CAC enzymes into a supramolecular compelx
where is NADH generated in the CAC (3)
isocitrate to alpha ketoglutarate by isocitrate dehydrogenase, alpha ketoglutarate to succinyl CoA by alpha ketoglutarate dehydrogenase complex, malate to oxaloacetate by malate dehydrogenase
where is FADH2 generated in the CAC
succinate to fumarase by succinate dehydrogenase
what is the formation of acetyl CoA from pyruvate in animal cells
irreversible
what are the two principal fates of acetyl CoA
metabolism by the CAC or incorporation into lipids
how is the activity of the pyruvate dehydrogenase complex controlled
tightly controlled allosterically and by reversible phosphorylation
how is the pyruvate dehydrogenase complex regulated allosterically
high concentrations of reaction products inhibit the reaction by informing the enzyme there is no need to metabolize pyruvate to acetyl CoA - acetyl CoA inhibits the transacetylase component (E2) and NADH inhibits the dihydrolipoyl dehydrogenase (E3)
what does acetyl CoA inhibit (pyruvate dehydrogenase complex)
transacetylase component (E2)
what does NADH inhibit (pyruvate dehydrogenase complex)
dihydrolipoyl dehydrogenase (E3)
how is the activity of the pyruvate dehydrogenase complex regulated (generally)
by reversible phosphorylation
how is the activity of the pyruvate dehydrogenase complex regulated (full)
active PDH uses kinase and ATP to turn inactive, release ADP. Inactive PDH uses H2O and phosphatase to turn active, release Pi.
at rest, what are the ratios of NADH/NAD+, acetyl CoA/CoA, and ATP/ADP
high
why are the ratios of NADH/NAD+, acetyl CoA/CoA, and ATP/ADP high at rest
promotes phosphorylation and inactivation of the complex by activating PDK
what happens during activity in CAC (biological conditions)
high ADP and pyruvate activate the complex by inhibiting the kinase. Ca2+ stimulates the phosphatase, enhancing pyruvate dehydrogenase activity
isocitrate dehydrogenase and alpha ketoglutarate dehydrogenase
allosteric enzymes that primarily regulate the rate of cycling in CAC. the first two enzymes that harvest high energy electrons in the cycle
what must the CAC intermediates be
rapidly replenished if any are used for biosynthesis
what do mammals lack
the enzymes for net conversion of acetyl CoA into oxaloacetate or other cycle intermediates
anaplerotic reaction
reaction that leads to the net synthesis, or replenishment, of pathway components
pyruvate carboxylase
catalyzes formation carboxylation of pyruvate to oxaloacetate. used in gluconeogenesis and is dependent on presence of acetyl CoA