Dietary Minerals and Electrolytes Overview
1/107
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
108 Terms
Dietary Minerals
Chemical Elements that cannot be formed by the body and are required in the diet.
Inorganic Minerals
Minerals that do NOT contain Carbon.
Mineral Charge
Minerals can carry a positive or negative charge which affects their interactions.
Mineral Combinations
Combinations of minerals can yield salts.
Mineral Requirements
Different minerals are needed in different amounts (range from less than 0.1mg to over 4g).
Mineral Interaction
Many minerals inhibit/activate each other's absorption.
Major Minerals
Minerals required in amounts greater than 100mg/day.
Trace Minerals
Minerals required in amounts less than 100mg/day.
Ultra-Trace Minerals
Minerals required in amounts less than 1mg/day.
Metabolic Functions of Minerals
Functions include enzyme cofactors, energy production, electrical conductivity, cell communication, electrolytes, fluid balance, bone and teeth health, protein structure, electron carriers, and oxygen transport.
Mineral Digestion
Many minerals in the diet are bound to proteins and require protein digestion in the stomach to release them.
Mineral Transport
Minerals are usually transported in their free form, complexed with other minerals (salts), or attached to protein carriers.
Body Storage of Minerals
The body has large stores of some minerals (e.g., Calcium and Phosphorus in bone) and can store trace minerals like iron and copper in organs such as liver, kidney, and spleen.
Calcium Sources
Sources include dairy, seafood with bones, some green vegetables, fortified foods, and supplements.
Calcium Adequate Intake
For ages 19-50 years: 1000 mg/day; for ages 51+ years: 1200 mg/day.
Calcium Absorption
Involves carrier-mediated active transport and paracellular diffusion.
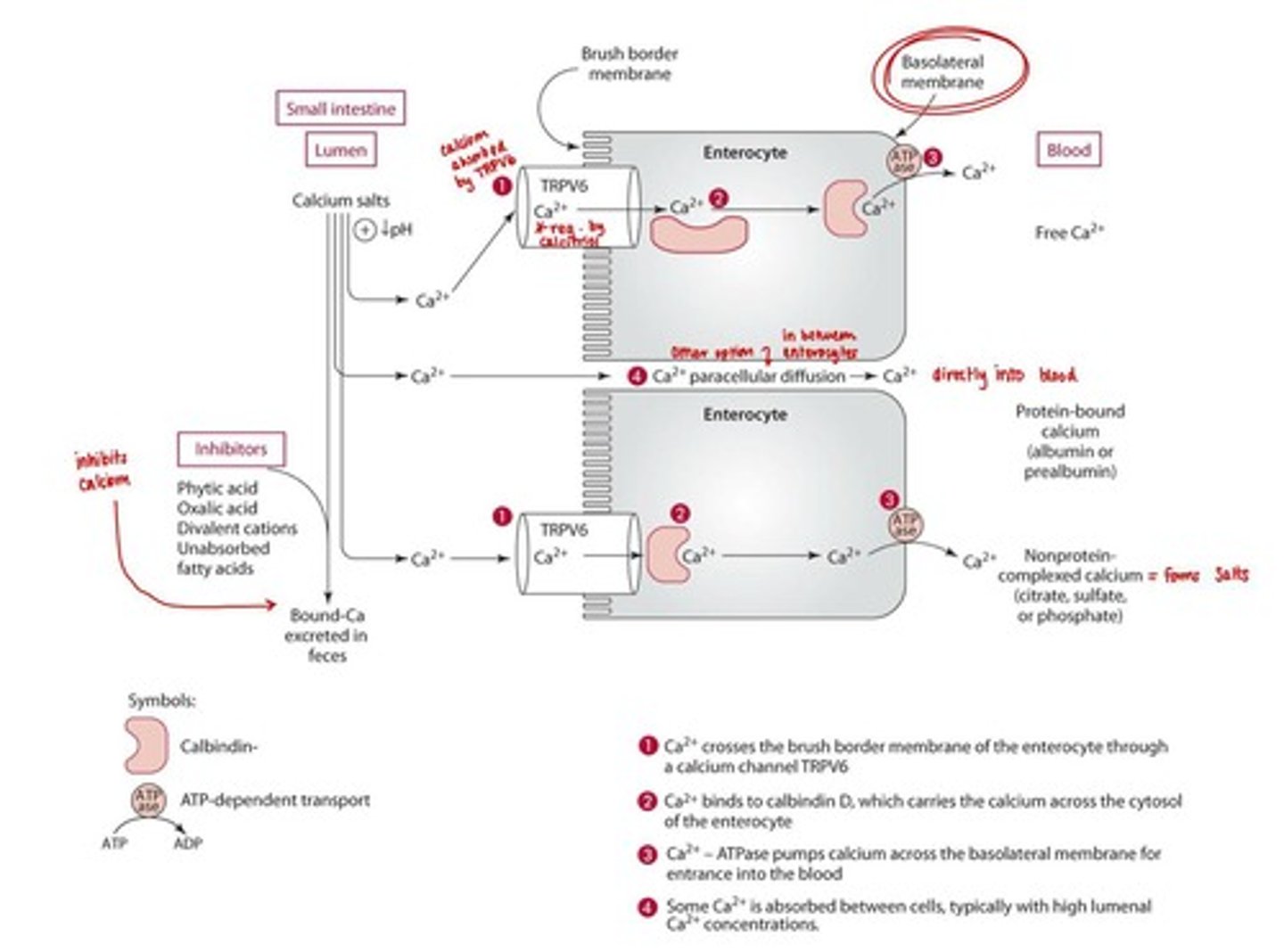
Calcium Absorption Enhancers
Vitamin D (calcitriol), sugars and sugar alcohols, and protein.
Calcium Absorption Inhibitors
Fiber, phytic acid, oxalic acid, excessive divalent cations (Zn, Mg), and unabsorbed fatty acids.
Calcium Transport
Calcium is transported bound to proteins (albumin and prealbumin), complexed with sulfate, phosphate, citrate, or free in the blood.
Calcium Regulation
Extracellular calcium concentrations are tightly regulated by PTH, calcitriol, and calcitonin.
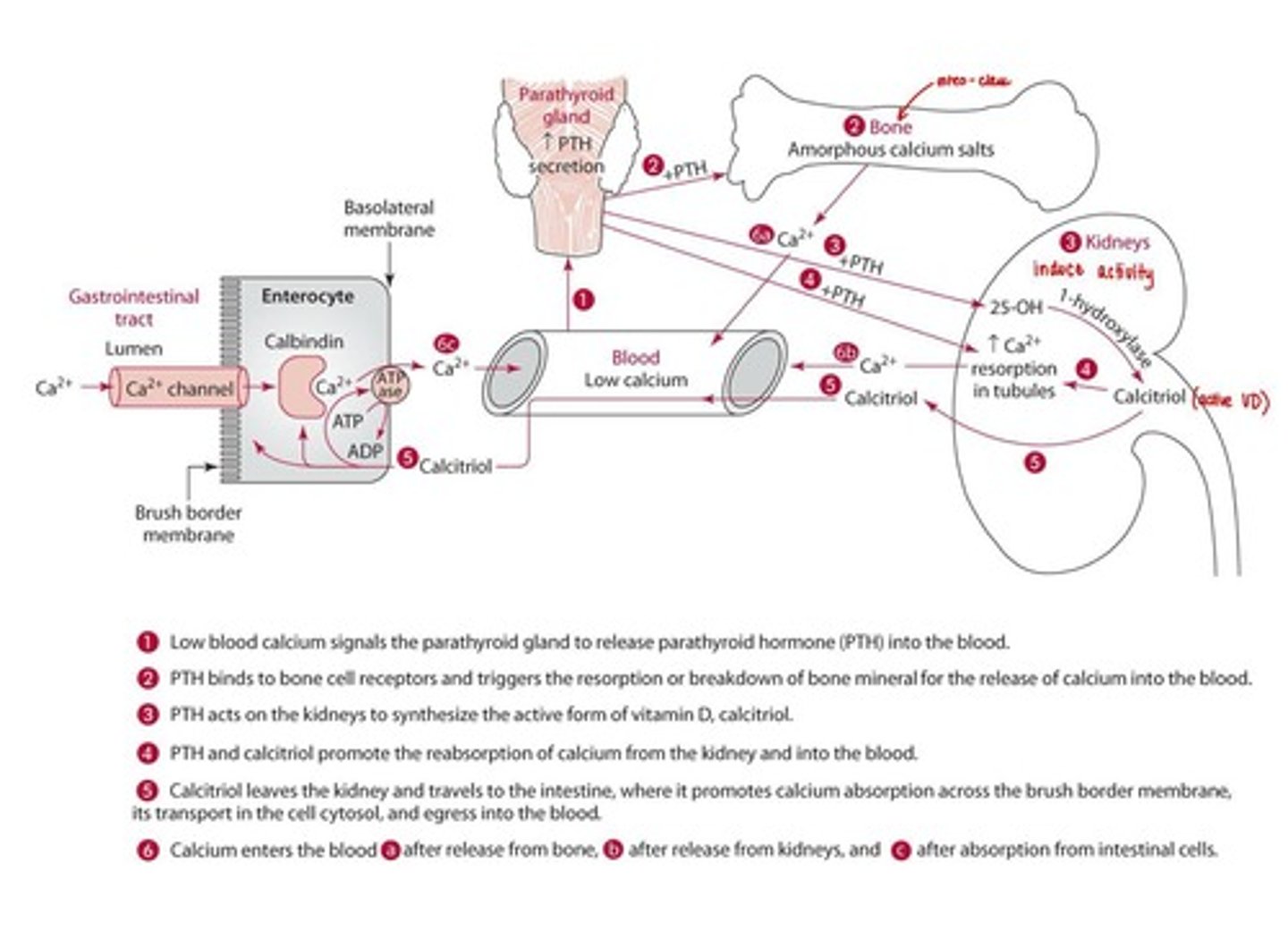
PTH
Parathyroid hormone that breaks down bone to release calcium into the bloodstream.
Calcium Tightly regulated by
PTH, Calcitriol, Calcitonin (opposite effect)
Bone mineralization
The process by which minerals are deposited in the bone matrix.
Blood clotting
The process by which blood changes from a liquid to a gel, forming a blood clot.
Muscle contraction
The process by which muscle fibers shorten and generate force.
Calcium deficiency
Rickets with a co-deficiency of vitamin D, Increased risk for osteoporosis, Hypertension, colon cancer, obesity
UL for Calcium =
2,500 MG/DAY (can cause kidney stones)
Phosphorus
A mineral essential for the formation of bones and teeth.
Sources of Phosphorus
Meat, poultry, fish, eggs, dairy, cola-type soft drinks, phosphate containing supplements.
RDA = 19+ years: 700mg/day
Recommended dietary allowance for phosphorus for individuals aged 19 years and older.
Digestion of Phosphorus
Hydrolyzed to inorganic phosphate. (Can be organic or inorganic)
Absorption of Phosphorus
Saturable, carrier-mediated active transport and diffusion.
Enhancers of Phosphorus absorption
Vitamin D and calcitriol.
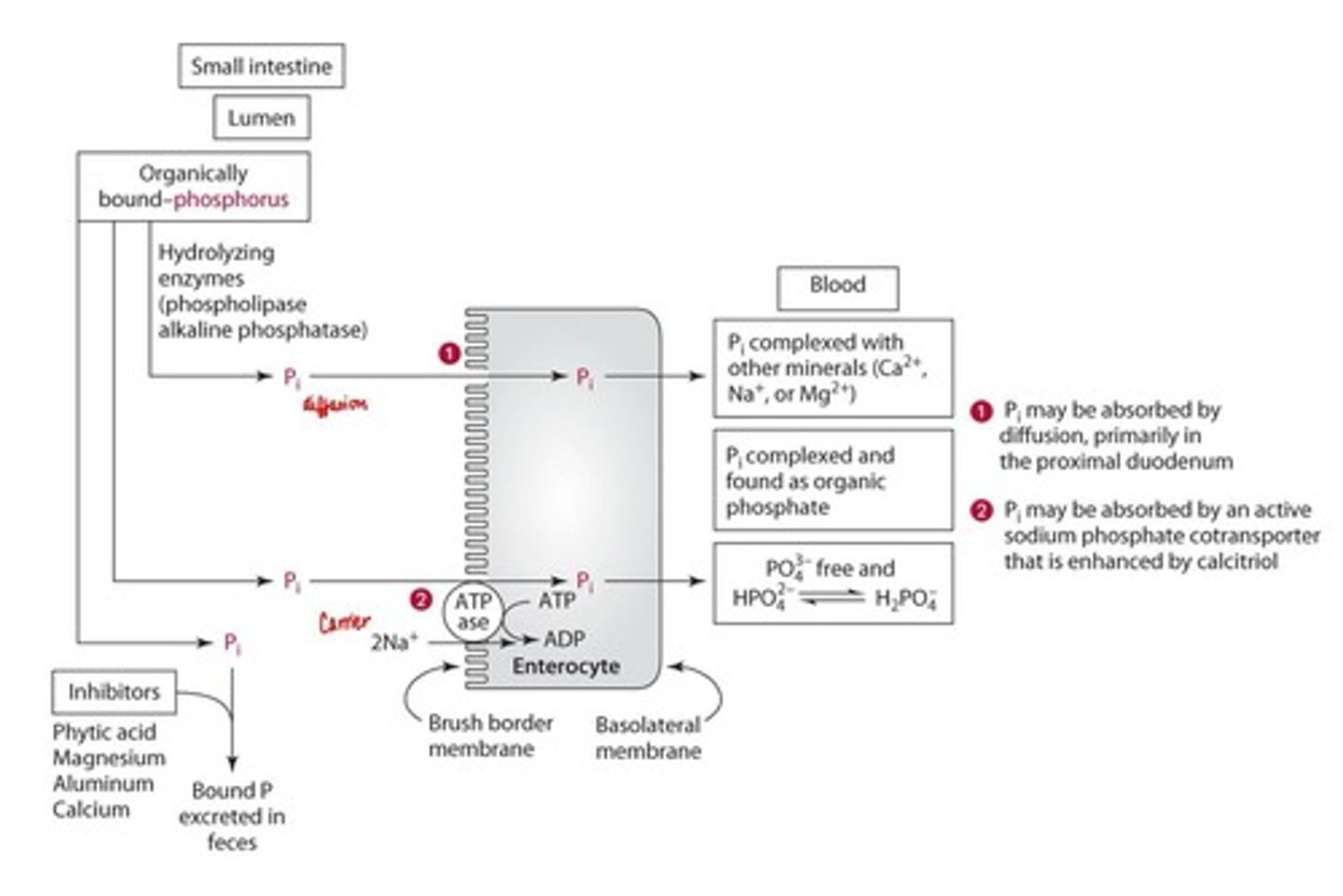
Inhibitors of Phosphorus absorption
Phytic acid (less bioavailable), excessive magnesium, aluminum, calcium.
Functions and Mechanisms of Phosphorus
Bone mineralization, nucleotide/nucleoside phosphates, phosphoproteins, phospholipids.
Deficiency of Phosphorus
Rare; renal patients at risk.
Toxicity of Phosphorus
UL: 9-70 years = 4g/day; >70 years = 3g/day.
Magnesium
A mineral important for many biochemical reactions in the body.
Sources of Magnesium
Nuts, legumes, whole grains, green vegetables, coffee, tea, cocoa.
RDA for Magnesium
Men: 400 mg/day (19-30 years), 420 mg/day (31+ years); Women: 310 mg/day (19-30 years), 320 mg/day (31+ years).
Absorption of Magnesium
Saturable, carrier-mediated active transport and simple diffusion.
Transport of Magnesium
50-55% free, 33% bound to protein, 13% complexed with negatively charged ions.
Enhancers of Magnesium absorption
Vitamin D, protein, carbohydrates.
Inhibitors of Magnesium absorption
Phytic acid, fiber.
Competitors of Magnesium absorption
Calcium, phosphorus, potassium.
Functions and Mechanisms of Magnesium
>300 enzyme reactions, structural cofactor, allosteric effector. Involved in glycolysis, Kreb's Cycle, Beta-Oxidation, nucleic acid synthesis, DNA transcription
Deficiency of Magnesium
Pure deficiency has not been reported; risk increased by malabsorptive disorders, excessive alcohol or diuretic use, parathyroid disease, burns increase risk.
Low intaes with CVD, type 2 diabetes, high BP
Toxicity of Magnesium
Possible impaired renal function; UL = 350 mg/day (non-food sources).
Sources of Iron
Heme iron from meat, fish, poultry; non-heme iron from nuts, fruits, veggies, grains.
RDA for Iron
Men: 8mg; Women: 18mg (premenopausal), 8mg (postmenopausal).
Digestion and Absorption of Iron
Heme iron is absorbed intact by heme carrier protein (hcp 1); non-heme iron is reduced to ferrous iron for absorption (main transporter DMT1...when iron is high DMT1 reduces, when iron is low DMT1 increases)
Enhancers of Iron absorption
Sugars (fructose, sorbitol), acids (ascorbic, citric), meat, poultry, fish, mucin
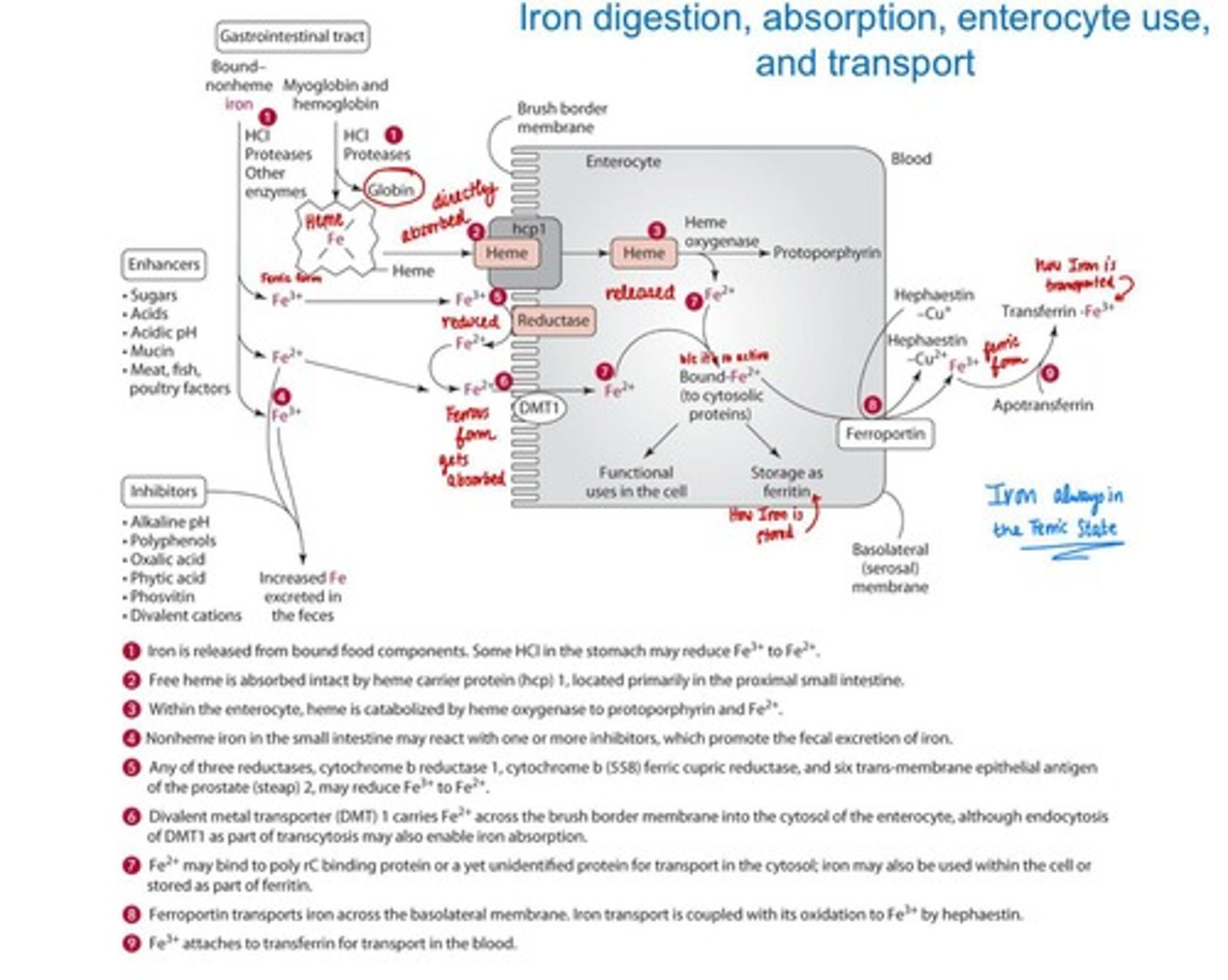
Inhibitors of Iron absorption
Polyphenols, oxalic acid, phytates, calcium, zinc, manganese.
Transport of Iron
Free Fe2+ binds to transferrin for delivery to tissues. Free Fe2+ can generate harmful free radicals
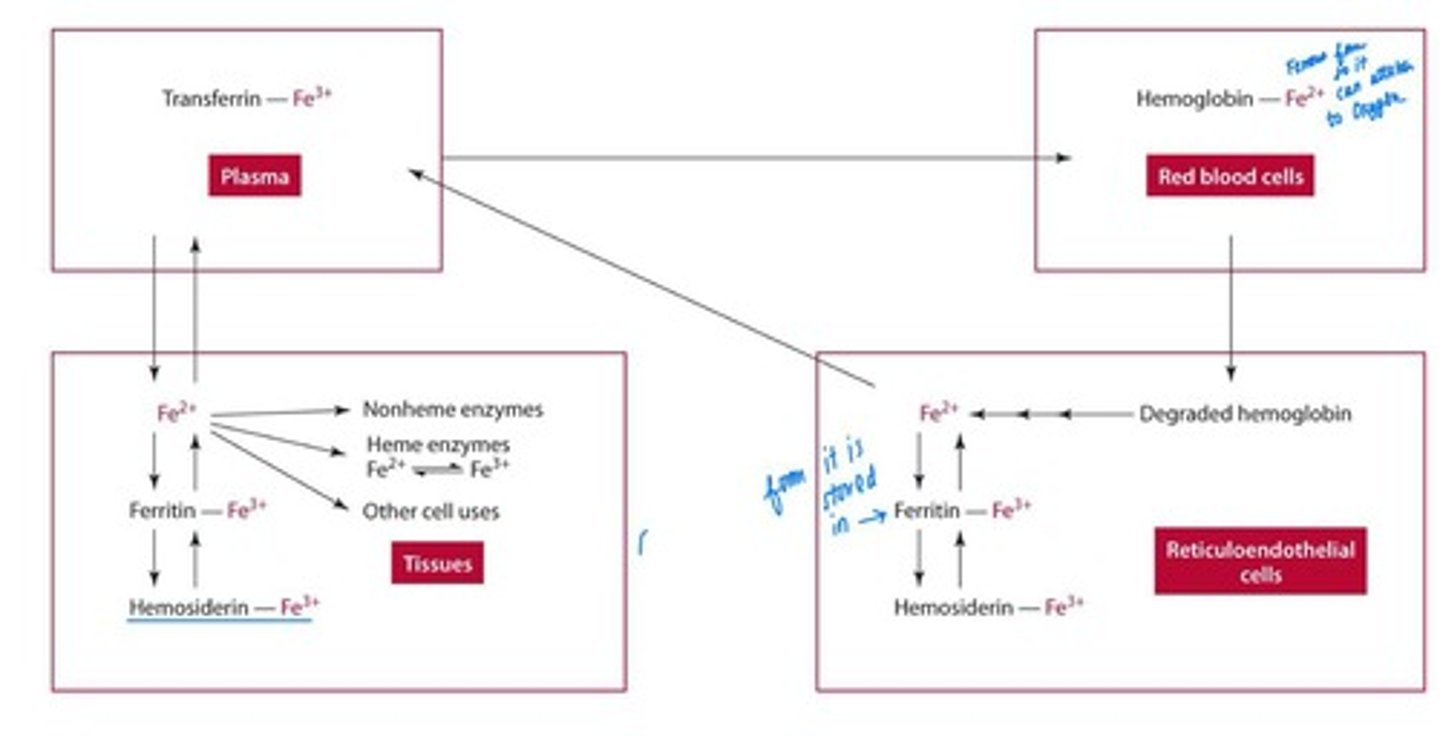
Storage of Iron
Stored in ferritin and hemosiderin at liver, bone marrow, spleen.
Functions and Mechanisms of Iron
Essential for hemoglobin, myoglobin, cytochromes, and various enzymes, peroxides
Deficiency of Iron
Hypochromic microcytic anemia; vulnerable groups include infants, adolescents, menstruating females, pregnant women.
Toxicity of Iron
TUL = 45mg; acute toxicity from accidental overload. Hemochromatosis (chronic iron overload)
Sources of Zinc
Red meats, seafood, poultry, pork, dairy, whole grains, vegetables.
RDA for Zinc
Men: 11mg; Women: 8mg; Pregnancy: 11mg; Lactation: 12mg.
Digestion of Zinc
Hydrolyzed from amino/nucleic acids in stomach and small intestine.
Absorption of Zinc
Carrier-mediated process and passive diffusion with high intake.
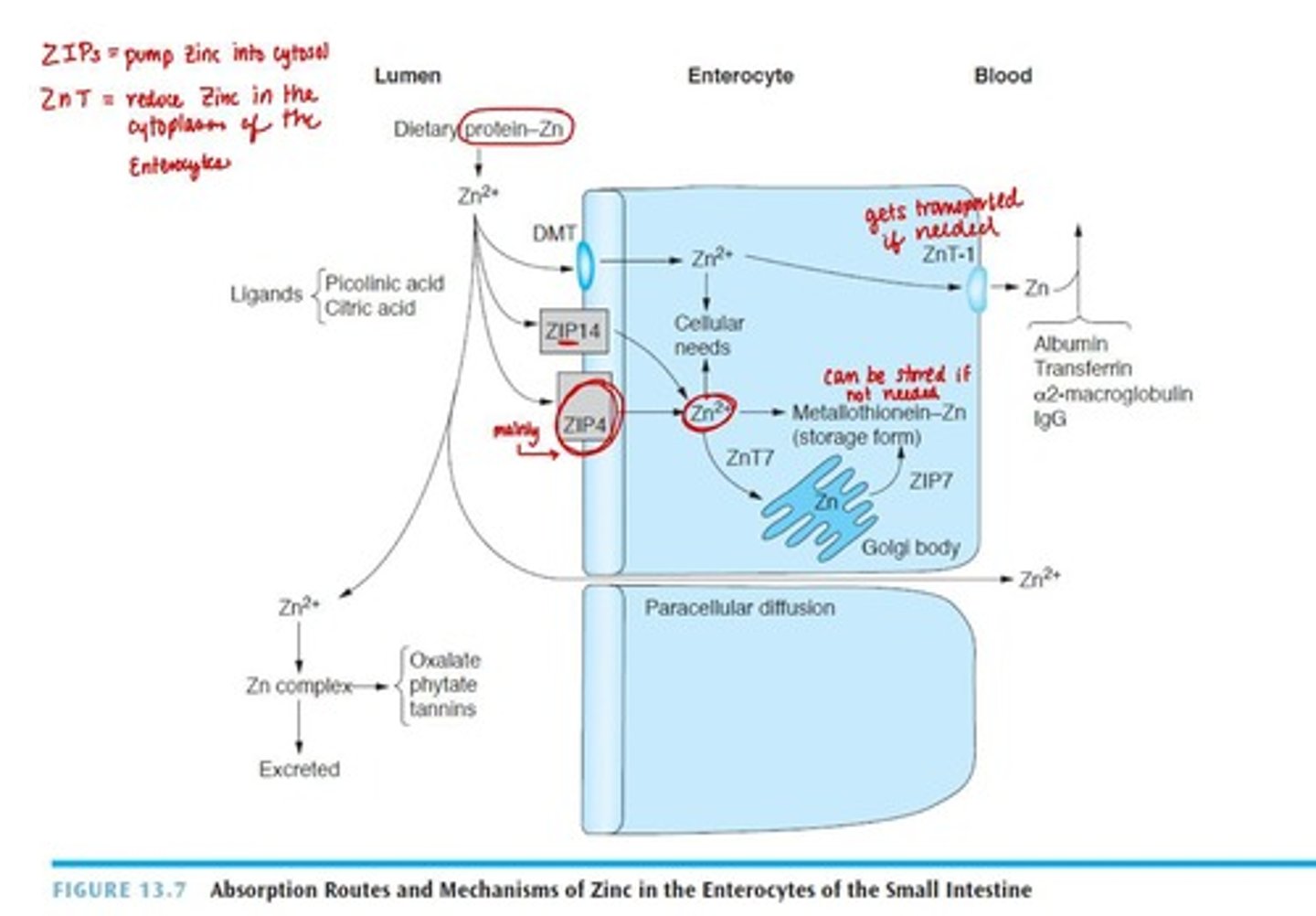
Transport of Zinc
Bound loosely to albumin in blood.
Enhancers of Zinc absorption
Ligands (chelators) like citric acid, picolinic acid, amino acids, acidic environment
Inhibitors of Zinc absorption
Phytate, oxalate, polyphenols, folate, iron, calcium.
Storage of Zinc
Found in all organic tissues, especially liver, kidneys, muscle, skin, bones.
Functions and Mechanisms of Zinc
Zinc-dependent enzymes present in over 200 enzymes, regulation of transcription, cell replication, bone formation, skin integrity, cell-mediated immunity, host defense, carbohydrate metabolism...ZINC Finger = proteins with a secondary structure due to the presence of a zinc atom linked throguh cysteinyl or histidyl residues
Deficiency of Zinc
Increased needs in elderly, children of low income, vegetarians, and those with alcoholism.
Acrodermatitis enteropathica (defect in ZIP4)
Toxicity of Zinc
UL = 40mg; high levels can cause copper deficiency.
Food Sources of Copper
Organ meats, shellfish, nuts, seeds, legumes, dried fruits.
RDA for Copper
Adults: 900ug; Pregnancy: 1,000ug; Lactation: 1,300ug.
Digestion of Copper
Bound to organic components in food, released by gastric HCl and pepsin.
Absorption of Copper
Small amount via stomach; primarily absorbed in small intestine.
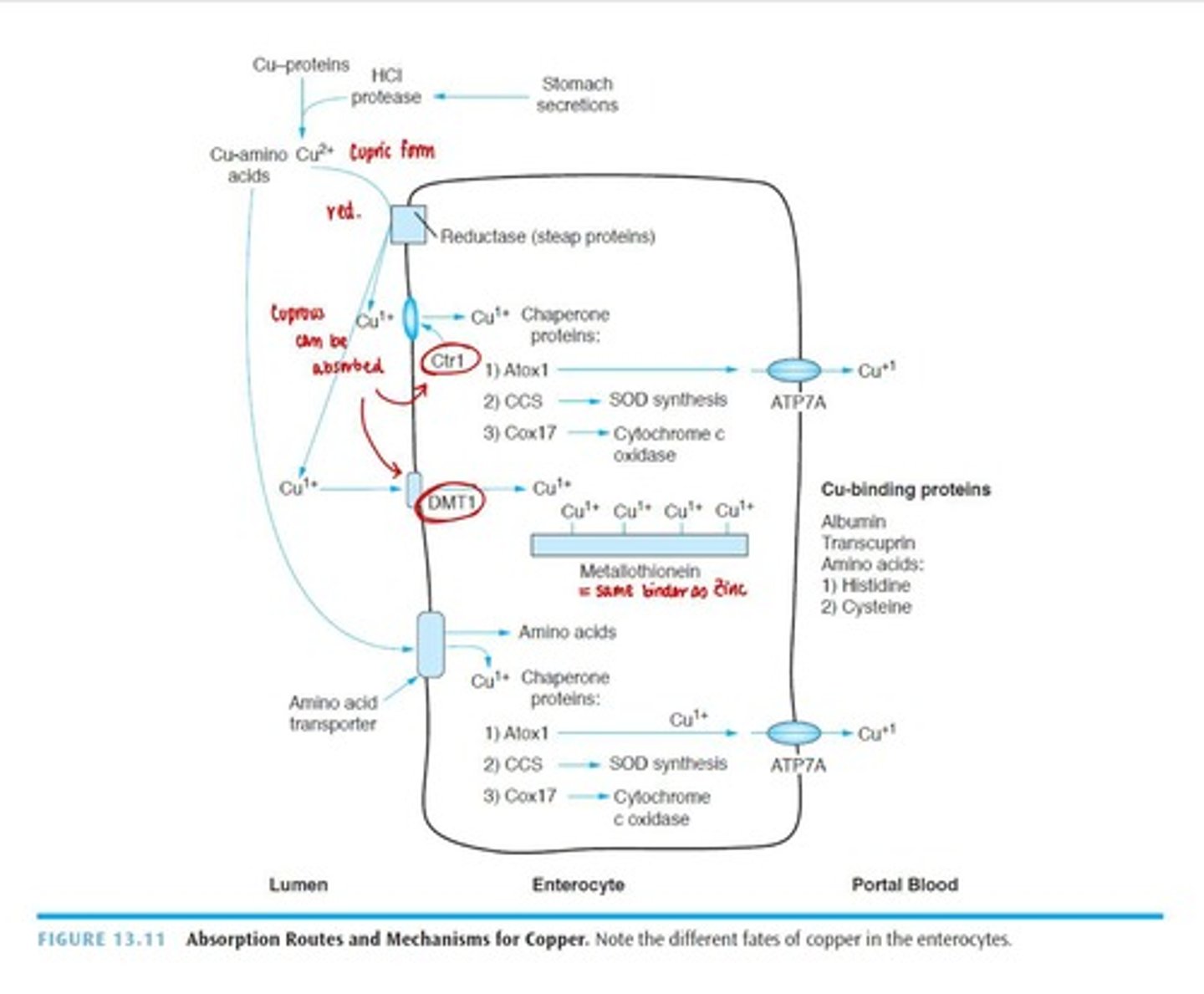
Enhancers of Copper absorption
Amino acids like histidine and cysteine.
Inhibitors of Copper absorption
Phytate, zinc, iron, excessive antacid ingestion.
Transport of Copper
Bound loosely to albumin in blood; binds to metallothionein in liver for storage.
Storage of Copper
Stored in liver, brain, kidneys, skeleton. Metallothionein - stores us to 12 copper atoms (high affinity for Zinc, higher for Copper)
Functions and Mechanisms of Copper
Ceruloplasmin for iron oxidation, superoxide dismutase as antioxidant, Cytochrome c Oxidase: ATP production
Deficiency of Copper
May be caused by excessive zinc consumption or kidney issues (nephrosis), GI malabsorption
Toxicity of Copper
UL = 10mg; Wilson's Disease is a genetic disorder characterized by copper toxicity.
Water
The universal solvent, is essential for life, able to dissolve different compounds
Properties of Water
Highly polar compound with positive and negative charges.
Water Intake
Males: ~2.5 Liters; Females: ~2.2 Liters of total beverages a day.
Sources of Water
Beverages, water from foods, small amount from metabolism (<10%).
Distribution of Water in the Human Body
60% of body weight is total body water; ⅓ is extracellular, ⅔ is intracellular.
Water Absorption
99.9% absorbed in the gut, primarily in the small intestine. Most (80-85%) is absorbed in the small intestine, the rest (15-20%) is absorbed in the colon
primarily absorbed through osmosis
Kidney Structure
Nephron is the functional unit, including Bowman's capsule and glomerulus.
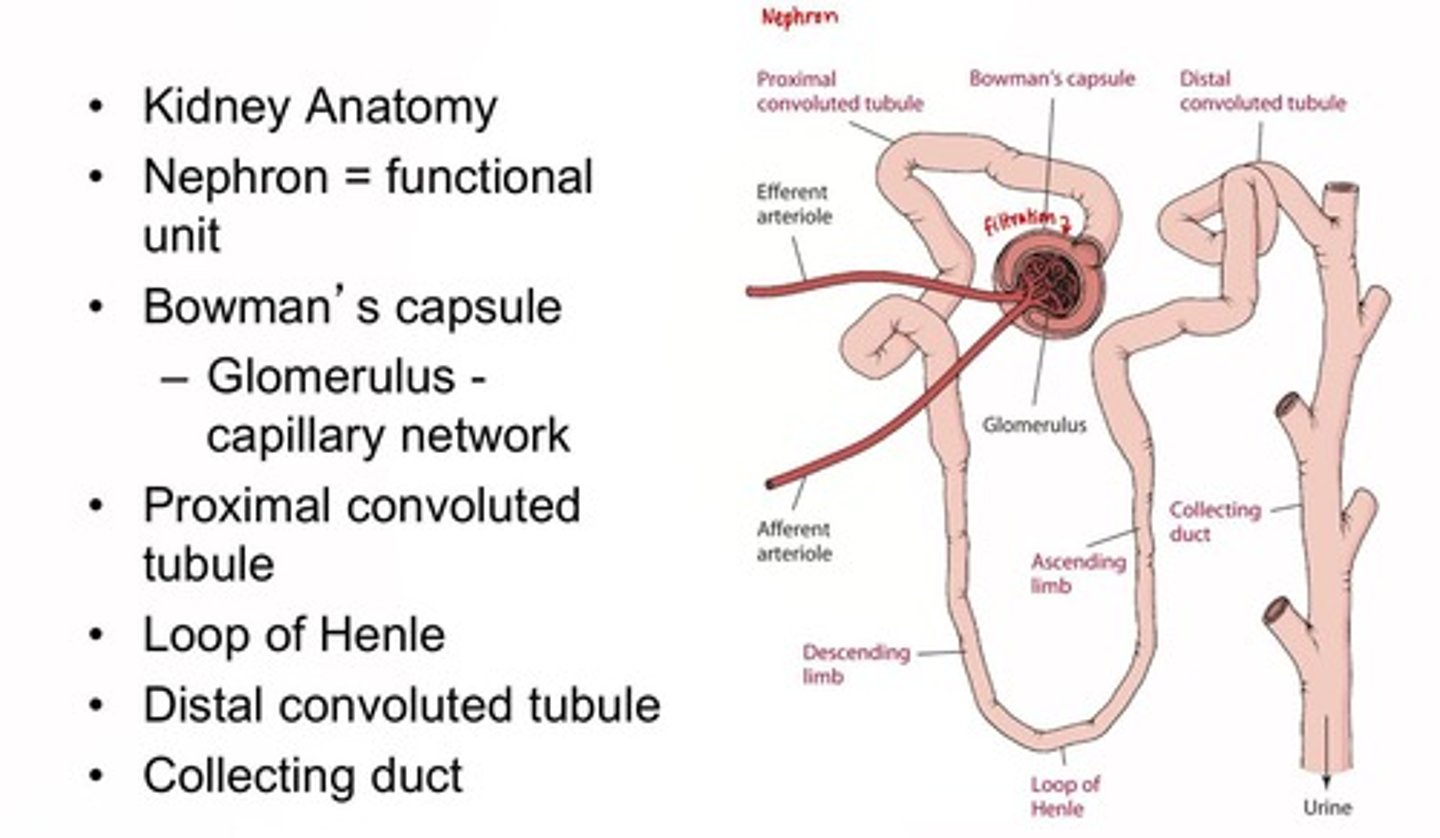
Kidney Role
Filtration and urine formation, filtering about 180 Liters of blood per day. Each liter of blood is filtered about 22-25 times in a single day
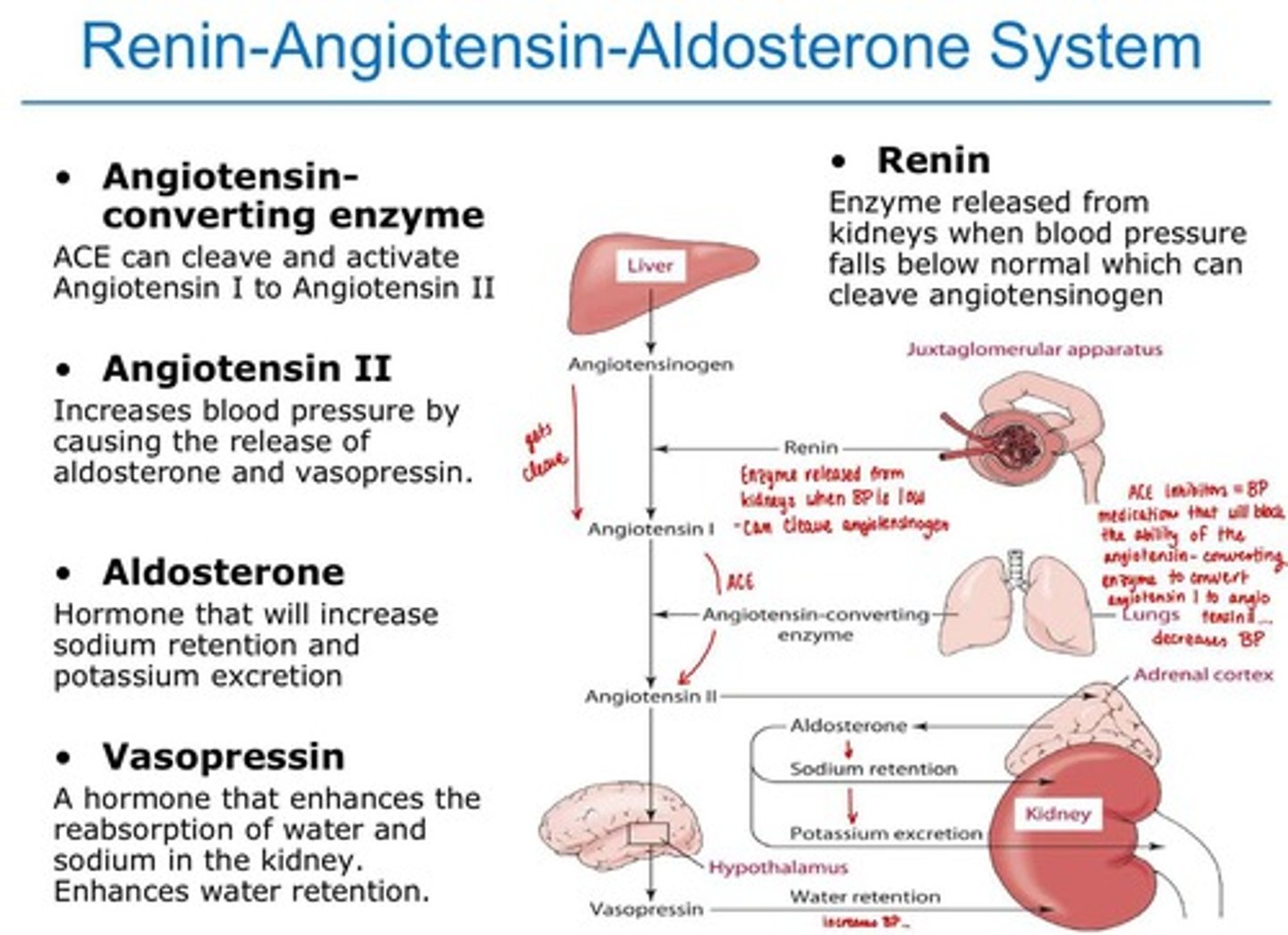
Endocrine Control: Renin-Angiotensin-Aldosterone System
Regulates blood pressure and fluid balance. **** Understand diagram!!!
Electrolytes
Chemicals that conduct electrical impulses in the body.
Sodium
Most abundant extracellular cation.
Adequate Intake of Sodium
1500mg/day; typical intake is 3.5g.
Food sources of sodium
canned, processed and frozen foods/meals; major source = table salt
UP/DV =
2400mg
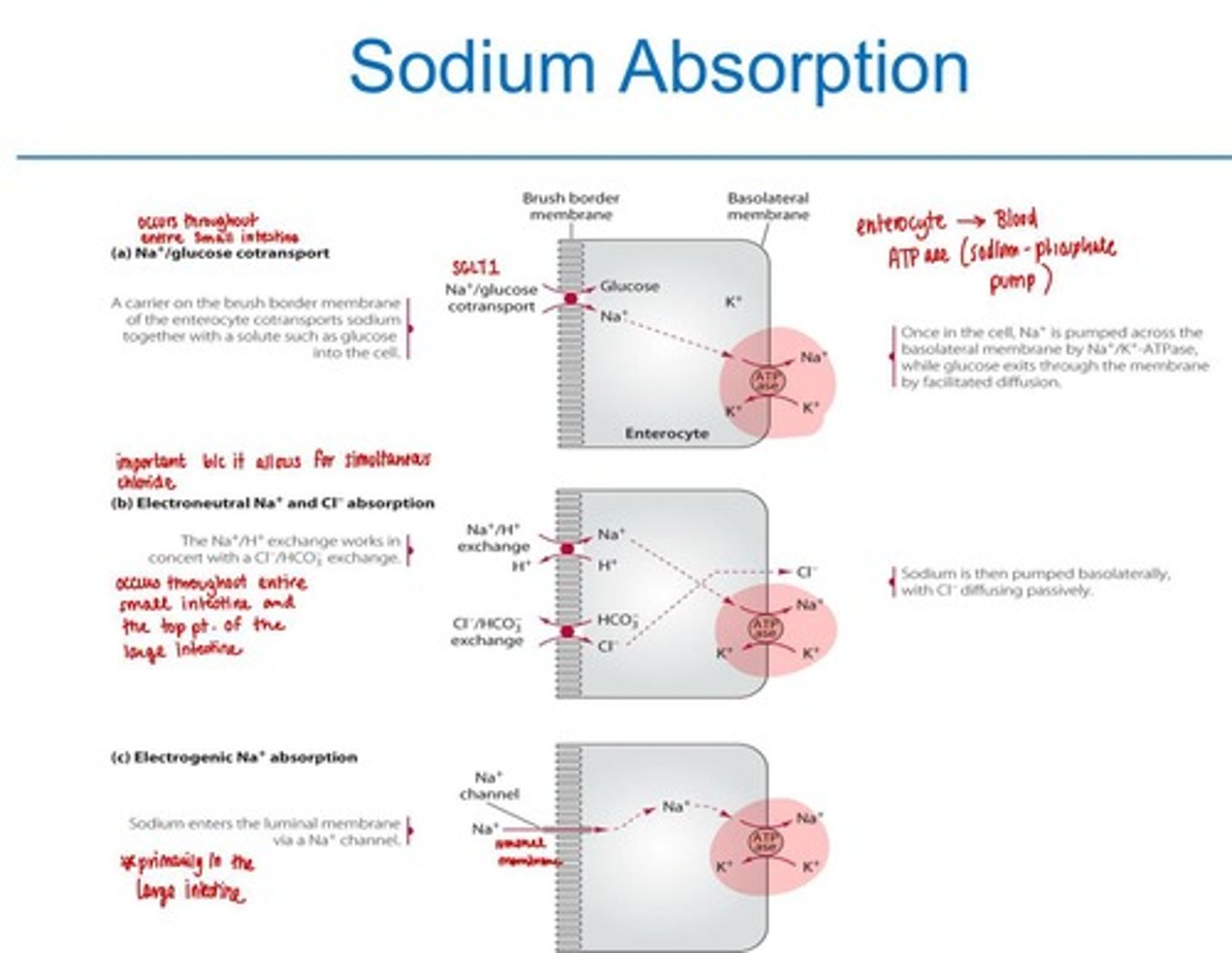
Sodium Absorption
3 pathways
1. The Na+/glucose co-transport - small intestine
2. electron-neutral Na+ and Cl- co-transport - small intestine and the proximal portion of the colon
3. electrogenic Na+ absorption - large intestine
* all 3 use ATPase to exit into the blood
deficiences of sodium
rare, associated with sweating
sodium is unique because
UP is LESS than DV; UL =2300mg/day
dietary sodium intake increases urinary calcium excretion
Pottasium is a
intracellular cation
sources of potassium
widespread in food
Absorption of potassium
>85% of potassium to be absorbed by passive diffusion or by a K+/H+ -ATPase pump
* stimulated by the hormoes insulin and catecholamines