Nucleic Acid Based Cancer Therapies 1
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
why is DNA a good drug target?
DNA possesses a number of nucleophilic sites :. target for electrophilic drugs
who has been targeting DNA for millions of years?
toxins that plants and bacteria release can target DNA
where is the major and minor groove in DNA?
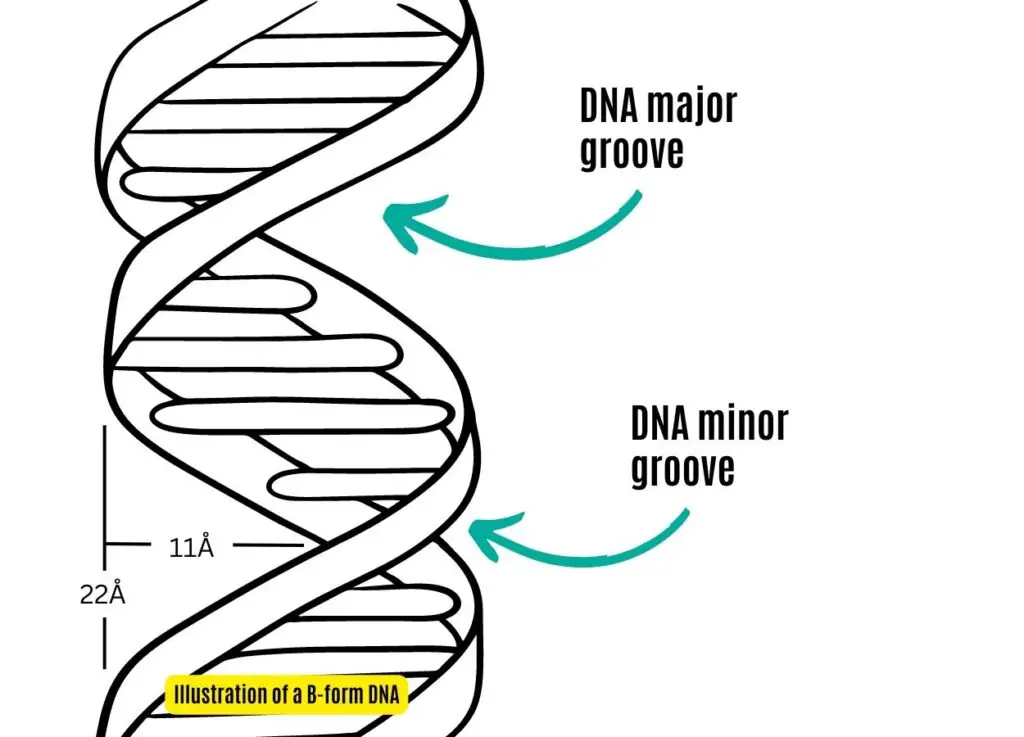
what are the different types of DNA interacting therapies?
DNA cleavage
intercalation
mono-adduct
topoisomerase adduct
interstrand cross link
intrastrand cross link
what do intercalating agents do?
drug drives a wedge between 2 strands
what do inter/intrastrand cross linkers do?
creates a covalent bond between 2 sections of DNA that aren’t meant to be cross linked
how can DNA repair produce resistance to DNA-interactive anticancer therapies?
if enzymes that repair DNA are in excess then the damage caused by anticancer drugs will be repaired :. counteracts action of drug
how does selectivity occur with DNA interacting agents?
mainly due to difference in growth rate of cancer cells compared to normal cells
could also come from reduced capability of cancer cells to repair DNA lesions compared to normal cells
some agents target specific DNA regions e.g. GC-rich sequences that may be prevalent in some tumour cells
may block action of key transcription factors that have been up-regulated in tumour cells
describe the DNA repair pathway and how this is relevant for DNA interactive agents
p53 = transcription factor that surveys genome and identifies mutations → makes decision on whether to promote expression of DNA repair enzymes or induce apoptosis
DNA repair pathways may become damaged in cancer cells as their genomes evolve to become increasingly mutated
what are the acute side effects associated with the DNA interactive class of anticancer agents?
alopecia
GI toxicity (mucositis and diarrhoea)
nausea and vomiting
reversible mone marrow suppression (most dangerous)
what are the side effects of long term use of DNA interactive anticancer agents?
gametogenesis is severely affected → sperm and ovarian tissue storage is recommended
increased incidence of acute non-lymphocytic leukemia and other cancers occurring later in life due to DNA damage caused by these treatments
what were the first DNA interactive agents? how were they discovered?
nitrogen mustards
serendipity
bombing of ship released mustard gas
doctors treating troops found lymphoid and myeloid suppression
realised it may be possible to use mustard gas to treat lymphoma and other leukemias since these are diseases of lymphoid cells
describe methylating agents as a class of anticancer drugs
adds methyl group to DNA
CH3+ + DNA → CH3-DNA
give 3 examples of methylating agents and where they act
dacarbazine
procarbazine
temozolomide
guanine in the major groove at the N7 and O6 positions
describe how dacarbazine works
it is a prodrug for the CH3 ion
in the body, dacarbazine is converted by enzymes into a methyldiazonium ion
methyldiazonium ion generates the CH3+ ion which methylates the DNA
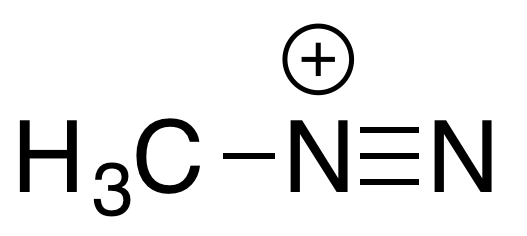
how is dacarbazine administered? why?
IV → IV bag must be protected from light because dacarbazine is photolabile
cannot be administered via injection into skin due to irritant properties of drug
what are the predominant side effects of dacarbazine?
myelosuppression
intense nausea and vomiting
how does procarbazine work?
it is a prodrug for the CH3 ion
in the body, procarbazine is converted by enzymes into a methyldiazonium ion
methyldiazonium ion generates the CH3+ ion which methylates the DNA
describe how temozolomide works
it is a prodrug for the CH3 ion
in the body, procarbazine is converted by CHEMICAL HYDROLYTIC CLEAVAGE (not enzymes) into a methyldiazonium ion
methyldiazonium ion generates the CH3+ ion which methylates the DNA
what is are the advantages of using temozolomide?
very good oral bioavailability
penetrates CNS :. used in brain tumours
how was temozolomide discovered?
in vitro screening
what does temozolomide’s action depend on at a biochemical level?
mismatch repair enzymes (MMRs) = DNA repair enzyme that detect the wobble base pair formed during replication of drug-modified DNA
when guanine is methylated, it looks like adenine to DNA repair enzymes :. removes original C paired to G and replaces it with T
this creates a kink in the DNA since G & T cannot pair → repetition of this causes MMR-induced strand breaks and causes arrest of cell cycle
how has resistance developed against temozolomide?
repair proteins have evolved → alkyltransferases (ATases) cleave the O6 and N7 modifications :. restores native guanine base
what does the response of a tumour to temozolomide depend on? how does this help treatment selection?
relative levels of MMR and ATases
can potentially screen patients to predict who would response: ideal = ATase -ve and MMR +ve
what are alkylating agents?
add >1 carbon to DNA
give examples of alkylating agents. how do they work?
carbinolamines
ET-743
alkylates N2 of guanine in minor groove using electrophilic attack :. adds bulky adduct that interferes with DNA processing
what makes ET-743 different from other DNA-binding agents available?
causes structural perturbation of the DNA molecule by bending it towards their site of interaction with DNA instead of away from it
pulls it rather than pushes it
how do cross linking agents work?
they contain 2 alkylating moieties separated by linkers
moieties are some distance apart
alkylates 2 nucleophilic functional groups either on same strand or opposite strand :. forming cross link
give examples of cross linking agents
nitrogen mustards → interstrand cross linkers in the major groove
aziridines
mitomycin C
nitrosoureas
what are the mechanisms of selectivity for nitrogen mustards?
targets faster growing cells
some cancer cells are not as good at repairing mustard adducts compared to healthy cells :. apoptosis induced
GC-selectivity
what are some of the potential mechanisms of resistance for nitrogen mustards?
multidrug resistance → MDR resistance (i.e. upregulation of p-glycoproteins)
increased conc. of glutathione in tumour cells → glutathione reacts with nitrogen mustards :. no longer electrophilic :. unable to launch electrophilic attack
up-regulation of repair processes where nitrogen mustards usually damage
what are the 2 types of nitrogen mustards?
aliphatic and aromatic
give an example of an aliphatic nitrogen mustard
chlormethine
this is the only aliphatic nitrogen mustard in use because they are highly reactive and able to attack other nucleophiles :. high toxicity profile
how does chlormethine work?
undergoes cyclisation in the body through chloride elimination
forms cyclic aziridinium ion
arizidinium ion attacks N7 of guanine in major groove of DNA
compound contains 2 chloride atoms :. process is repeated with second chloroethyl group → attacks second guanine N7 on opposite strand
causes interstrand cross link that locks 2 strands together
why are aromatic nitrogen mustards preferred over alipahtic?
milder alkylating agents
aromatic ring acts as an electron sink :. withdraws electrons from nitrogen atom :. discourages arizidinium ion formation
less reactive :. makes travel to tumour site better → less toxicities observed
how do aromatic nitrogen mustards work?
central nitrogen electron pair is delocalised with pi electrons of the aromatic ring :. not sufficiently basic to form a cyclic arizidinium ion
:. alkylation most likely occurs via SN1 mechanism → normal carbocation formation caused by chloride ion ejection = rate-limiting step
occurs for first Cl then repeated for second Cl
attacks N7 guanine on one strand followed by second :. interstrand cross link
how are aromatic nitrogen mustards administered?
orally since they can reach target site without interacting with other nucleophiles = big advantage
give an example of a conjugated aromatic nitrogen mustard
melphalan
contains carboxylic acid giving it greater selectivity since it increases uptake in cells where protein synthesis is occurring faster
what are the dosing intervals like for melphalan?
every 6 weeks
bone marrow toxicity is delayed :. have to wait longer to see effects
how do aziridines work? name an example
instead of forming aziridinium ion, they have aziridine ring already incorporated into its structure
e.g. thiotepa
compare aziridines with nitrogen mustards
ring opening of aziridines with nucleophiles is slower compared to aziridinium ions in nitrogen mustards
which 3 moiety components of mitomycin C are essential for its activity?
quinone
arizidine
carbamate
describe the selectivity of mitomycin C
selective for hypoxia
describe how mitomycin C is administered
IV
6 week intervals → delayed bone marrow suppression
what is the biggest benefit of using nitrosoureas over other cross linkers?
high lipophilicity compared to other cross linkers :. can cross CNS :. can be used in brain cancers