Corrosion and its prevention
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What is corrosion?
The gradual destruction of metals due to chemical reactions with substances in the environment.
What is rusting?
The corrosion of iron or steel when exposed to oxygen and water.
What is the word equation for rusting?
Iron + Oxygen + Water → Hydrated Iron(III) Oxide (Rust)
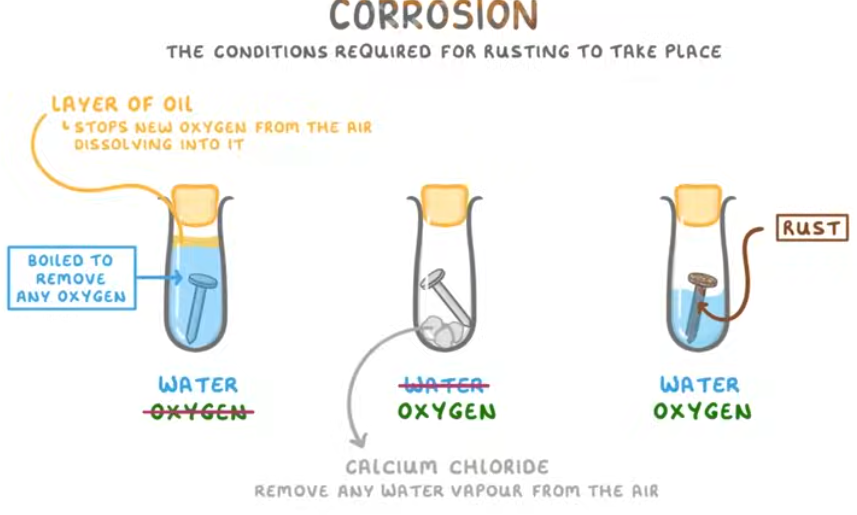
What are the conditions required for rusting?
Both oxygen and water must be present.
How does rust weaken iron?
Rust is soft and flaky, so it crumbles away, exposing more iron to further corrosion, until the iron completely degrades and disspears
Does aluminium corrode in the same way as iron?
No, aluminium forms a protective aluminium oxide layer, preventing further corrosion.
How do barrier methods prevent corrosion?
They block oxygen and water from reaching the metal surface.
What are three common barrier methods?
Painting/coating with plastic – Used for cars, fences, bridges.
Oiling/greasing – Used for moving parts like bike chains.
Electroplating – Coating the metal with a less reactive metal (e.g., chromium, tin).
What is sacrificial protection?
Attaching a more reactive metal (e.g., zinc or magnesium) to iron, which corrodes instead of iron.
Why does sacrificial protection work?
The more reactive metal oxidises first, preventing iron from corroding.
Where is sacrificial protection used?
✅ Ship hulls
✅ Underground pipelines
What is galvanising?
Coating iron or steel with zinc to prevent rusting.
How does galvanising prevent corrosion?
✅ Zinc acts as a barrier (stopping oxygen and water from reaching iron).
✅ Zinc provides sacrificial protection if the coating is scratched.
how to investigate rusting of iron with three test tubes
.