Chapter 6- Ionic and Molecular Compounds
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
transfer of electrons
all atoms want to be stable = all want 8 valence electrons
Octet rule
atoms wil gain, lose or share electrons to get to 8. doesn't happen with He because it's stable with two
Electron-Dot Diagrams (Lewis Dot Diagrams)
Shows the valence electrons distributed around the elements symbol
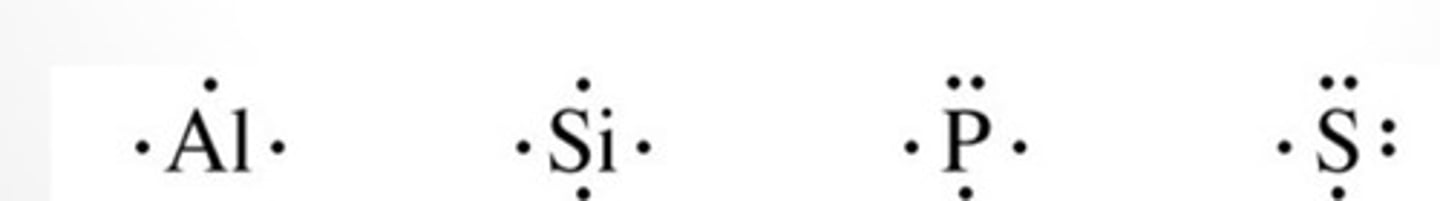
Metals
Lose elections and have a positive charge because they have more protons then electrons
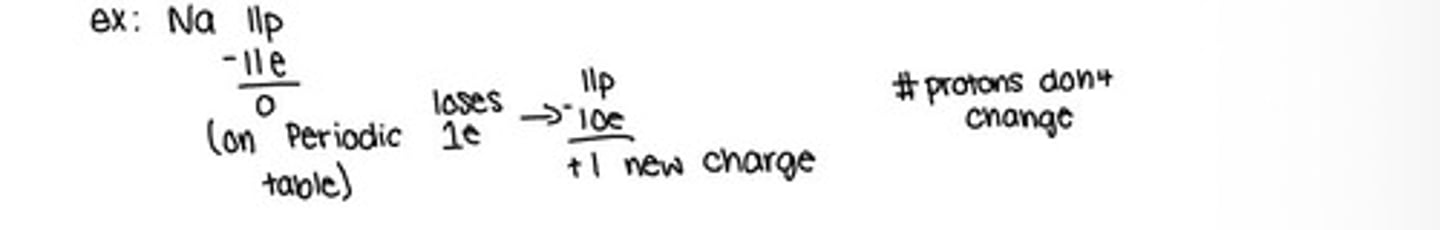
Non-metals
Gain electrons and have a negative charge because they have more electrons than protons

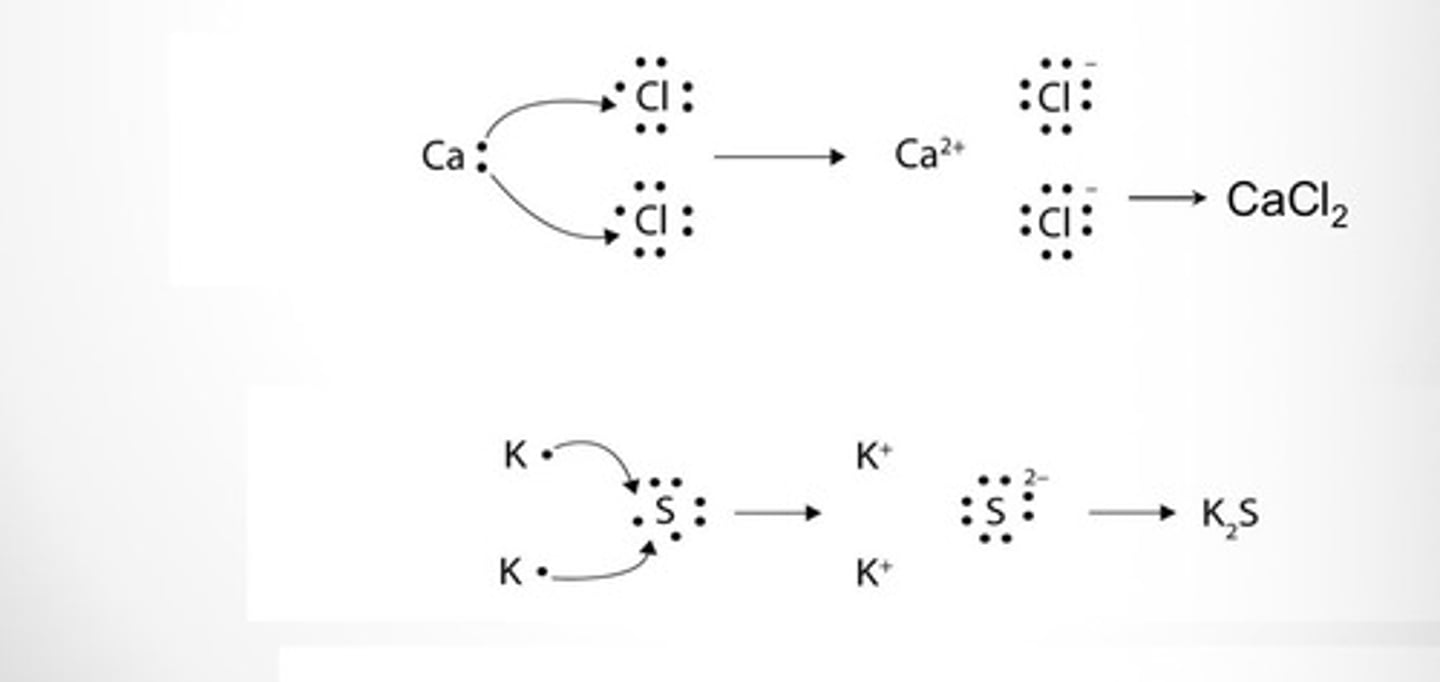
Ionic Bonds
Form when a metal gives away electrons to a nonmetal forming ion
Ion
An atom with a charge (positive or negative)
Metals (ions)
Positive ion (cations)
Cations
positive symbol with a superscript of the charge after the symbol. Named after the element.

Cations formation
Formed by the lose of one or more electrons
Sodium ions (Na+)

Anions
Atoms with a negative charge
Anion formation
Nonmetals achieve an octet arrangement by gaining electrons with the electron configuration of their next noble gas
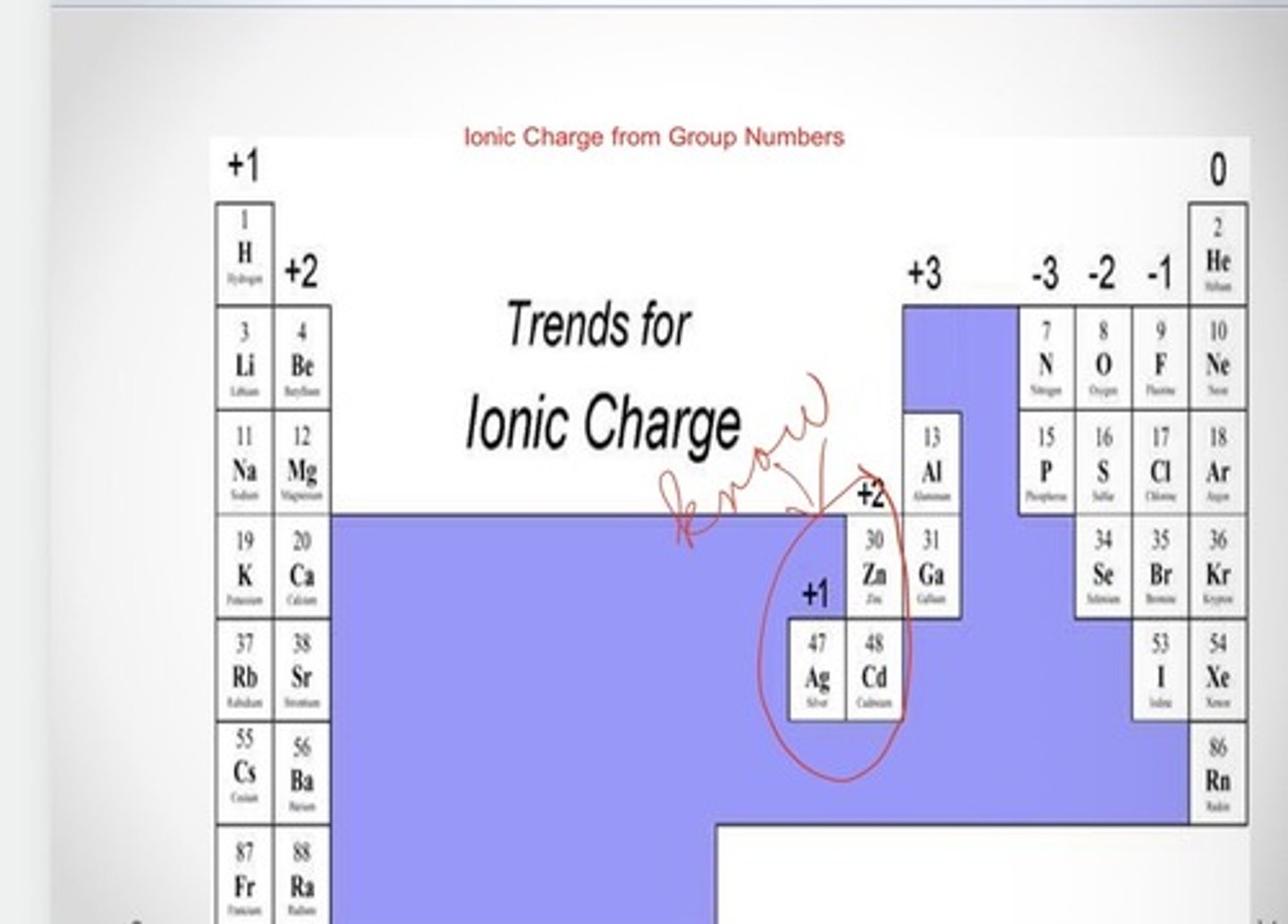
Ionic charge from group numbers
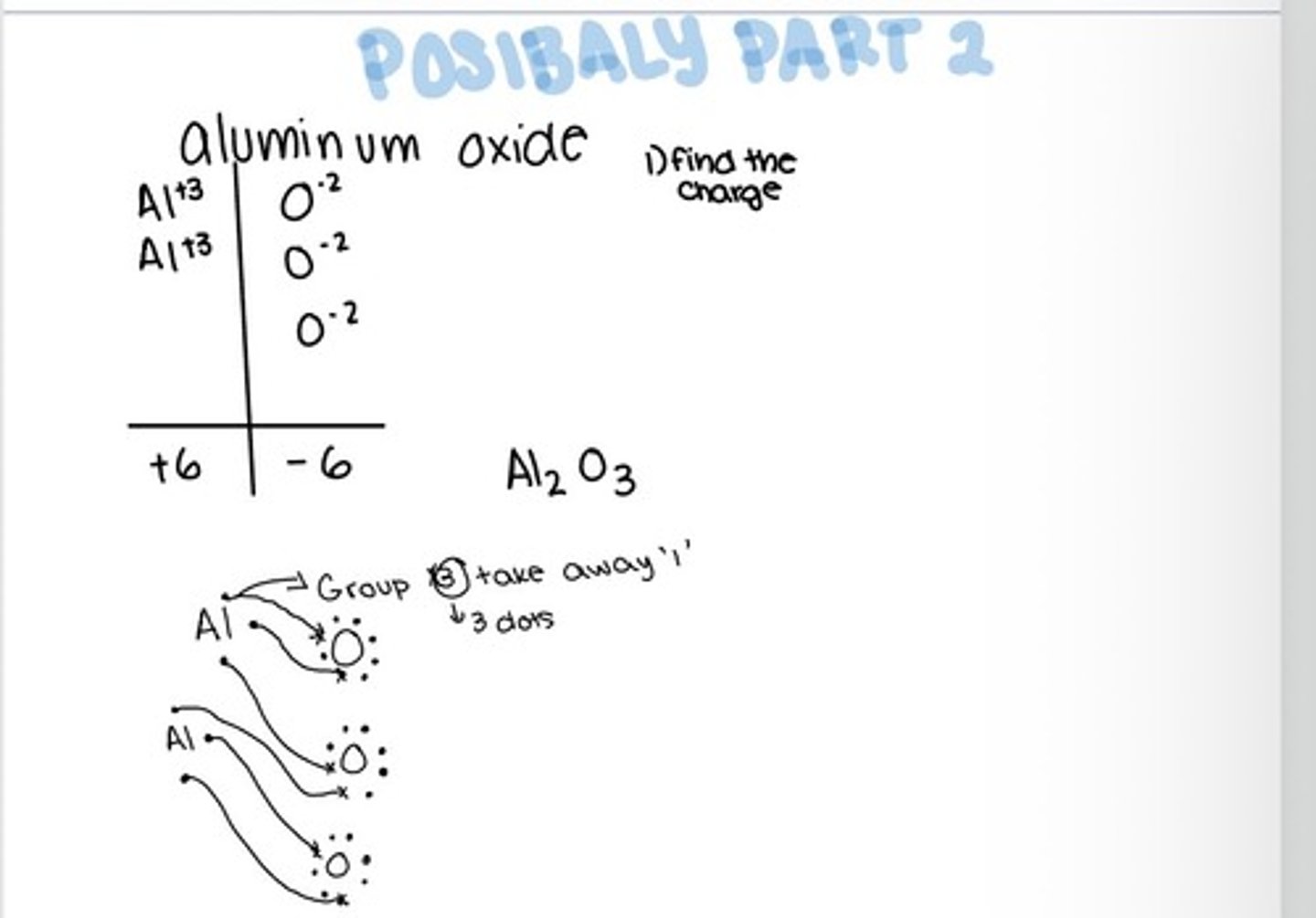
Ionic Compounds
Consist of metal cation (+) and nonmetal anion (-) and have attractions called ionic bonds between positive and negative charged ions
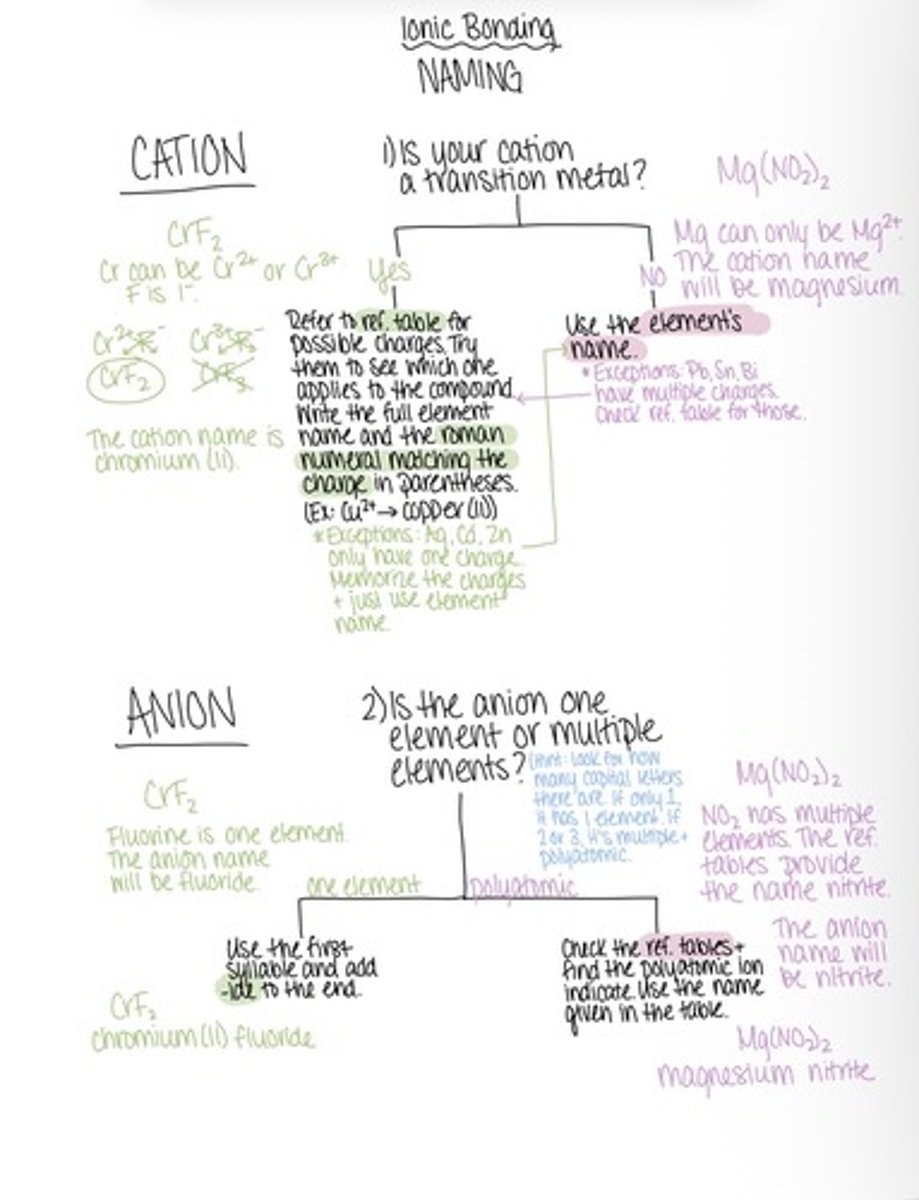
To name a ionic compound
Cation is named first and names the element. The anion is named second and changes the end of to -ide.
Ionic compounds consist of
Positive and negative charged ions and is electrically neutral. Net charge of 0
3 different ways
Chart, dots, crisscross
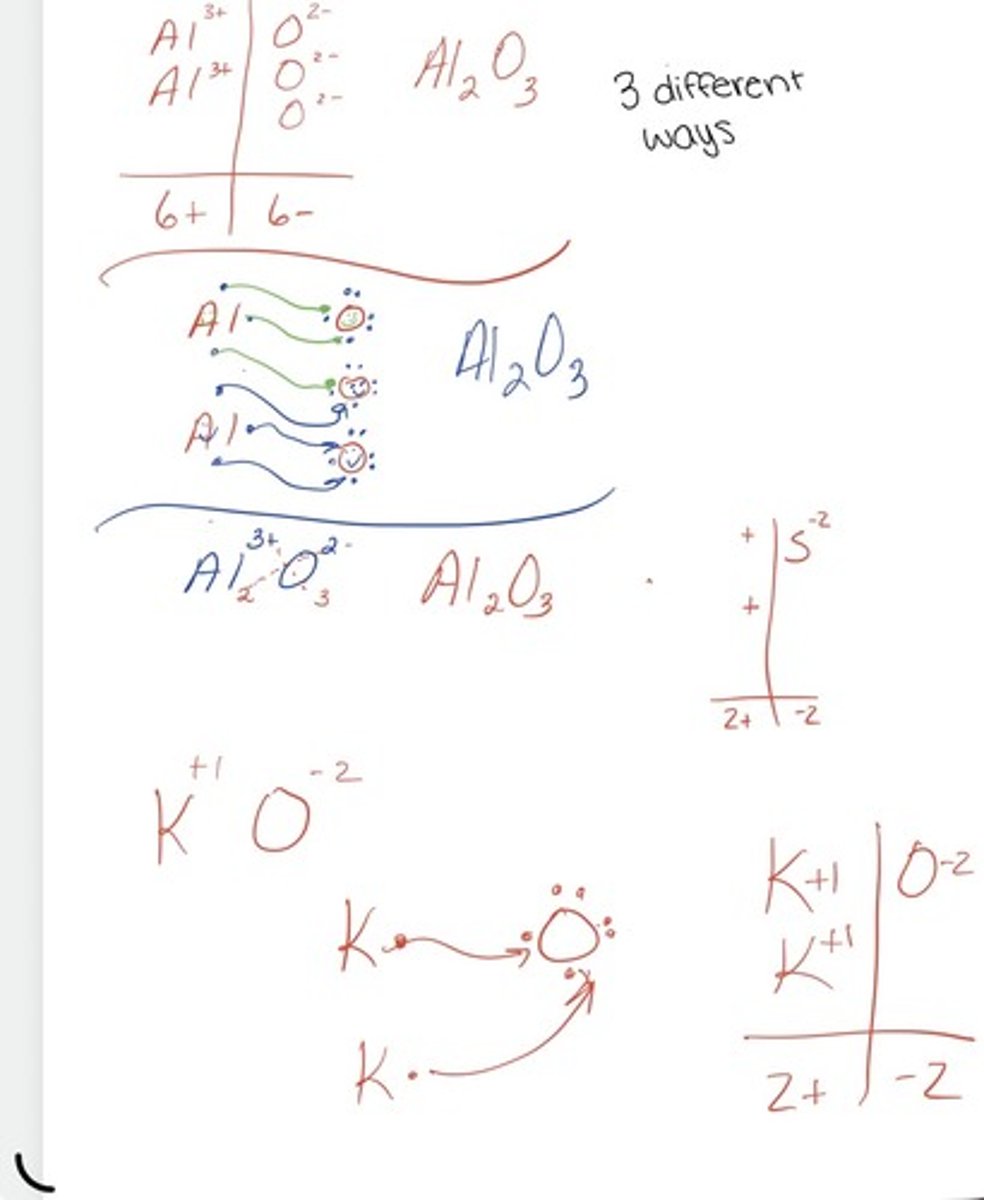
chemical formula of compounds
Gives the element symbols and a subscript telling how many each atom have
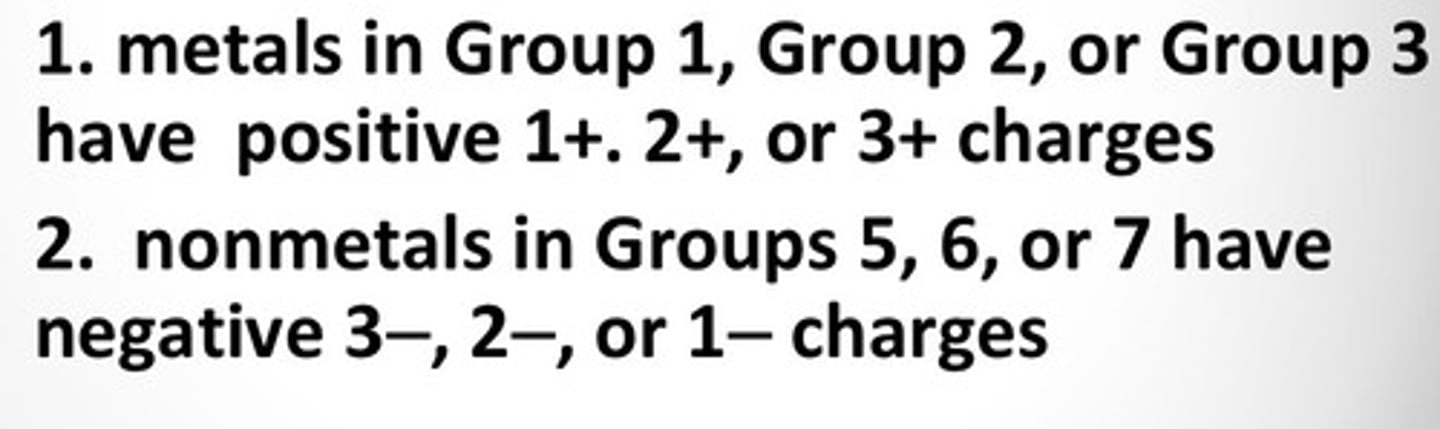
Example of the chemical formula
NaCl

Transition Metals (Groups 3-12)
Can form two or more positive ions with different ionic charges
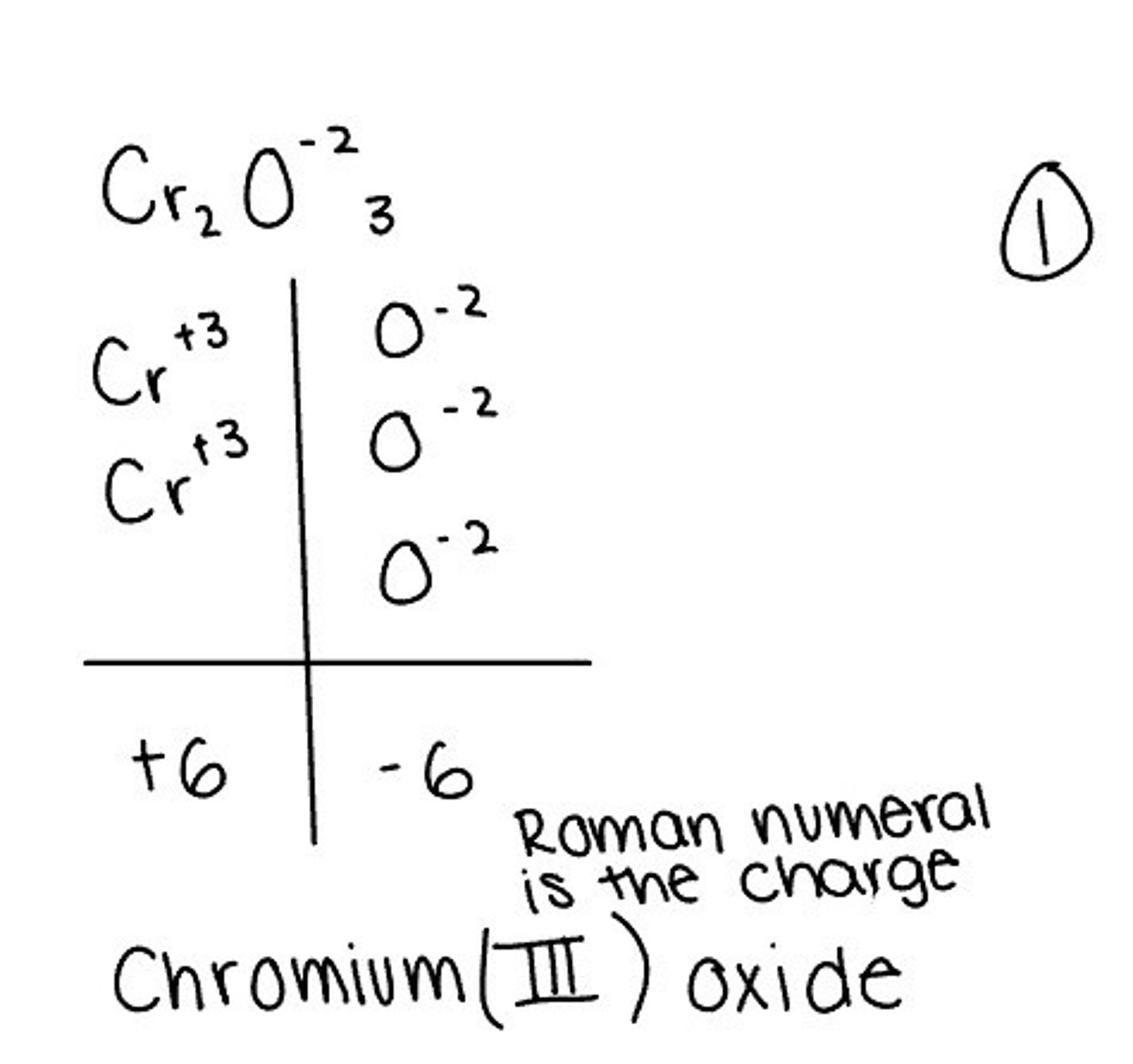
Names of compounds with variable charge metals
Transition metals with two different ions use a Roman numeral (Roman Numeral is the charge) ex: FeCl2= Iron(II) chloride
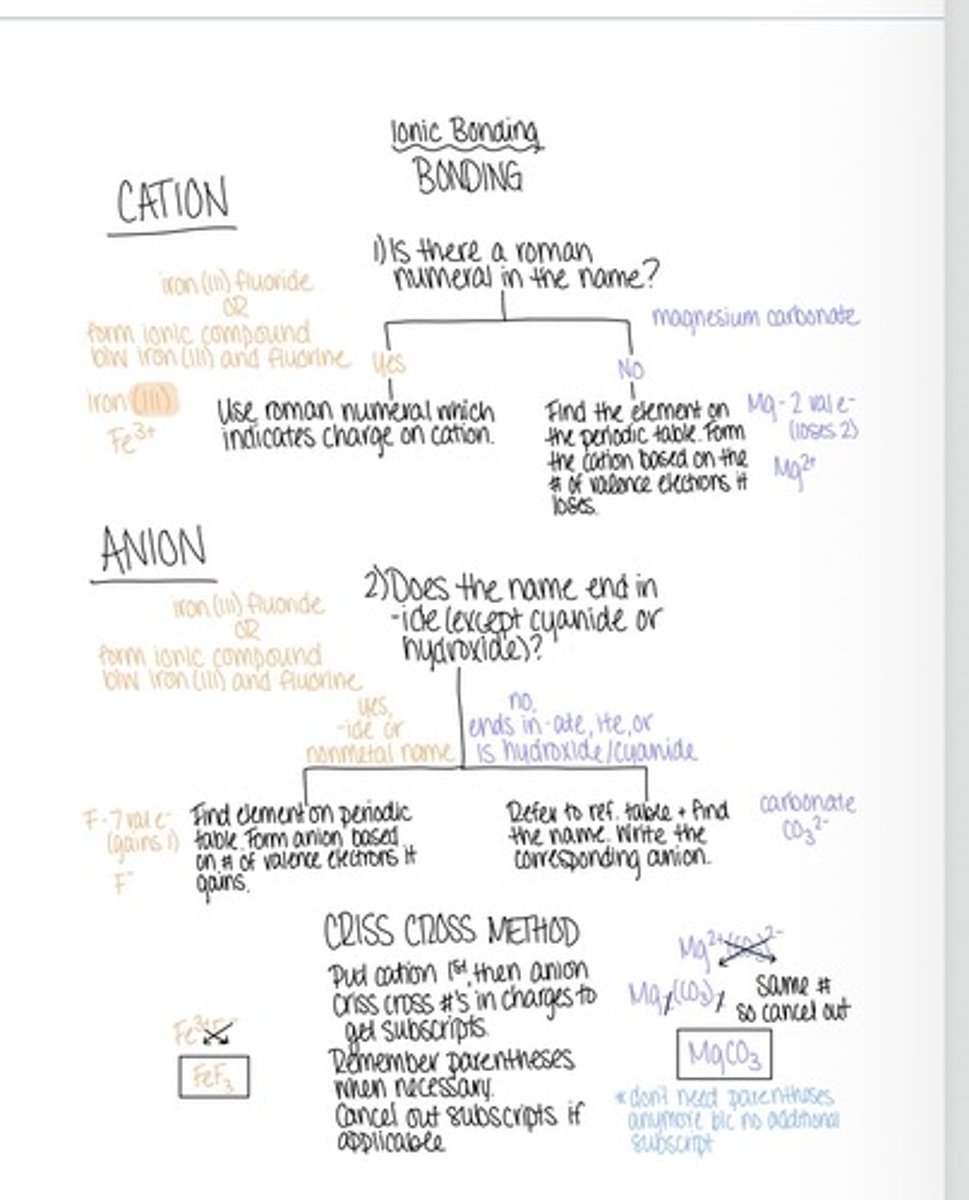
Ionic bonds
Holds ions together in an ionic compound
Formulas for ionic compounds
Dot method, crisscross and this

polytomic ion
A group of bonded atoms (non-metals) that has an overall ionic charge
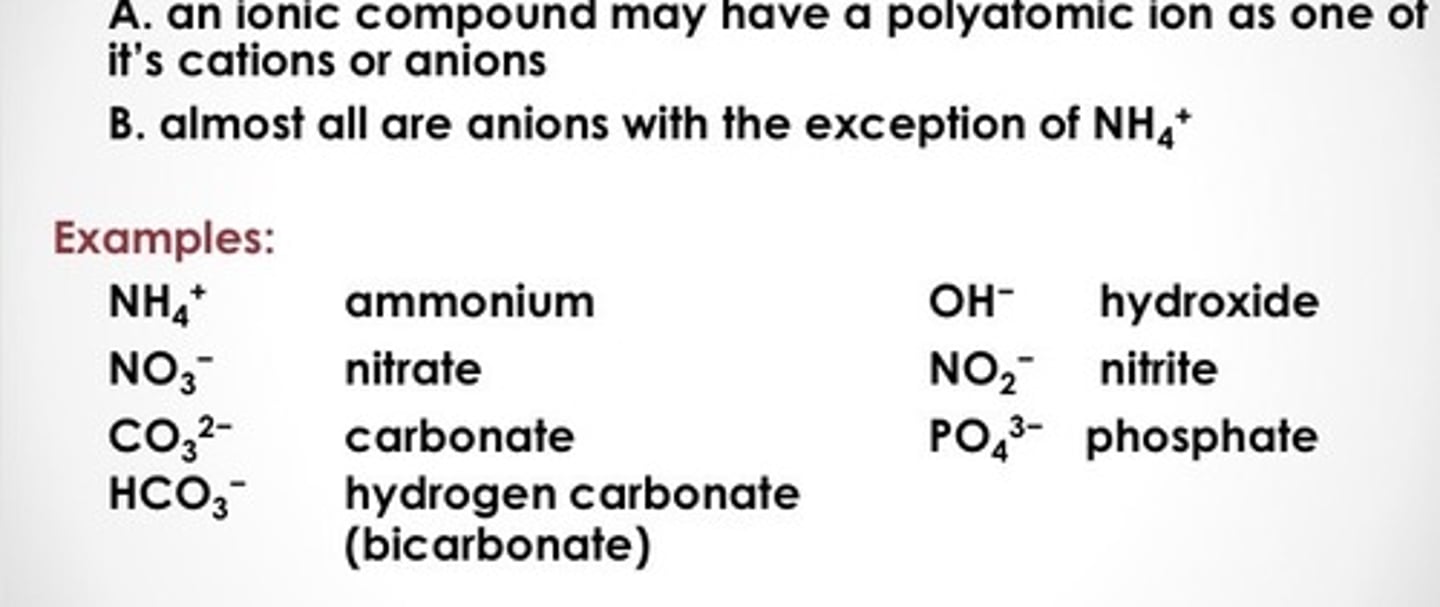
More names of polyatomic ions
Ends with ate, ite or start with bi

Ionic bonding naming
Ionic bonding bonding
Molecular compounds
Inorganic compounds that form a covalent bond where the valence electrons are shared
Molecular formulas
Shows the number of atoms that a molecule contains. A molecular formula is composed of two elements. Each and nonmetal prefixes are used to identify the number of atoms in each element.
Possibly part 2