BIOB10 - final exam prep
1/395
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
396 Terms
What if large amounts of unfolded or misfolded proteins are accumulating in the ER?
Cells have evolved a plan of action = Unfolded Protein Response (UPR), which helps relieve the stress on the ER caused by defective proteins.
what happens during UPR
• More chaperones are made and more proteases that degrade defective proteins are made.
• Synthesis (translation) of new proteins not involved in the UPR is decreased.
Activation on UPR is based on
chaperones binding to sensors decreasing. This is because their binding to misfolded proteins in the ER increases.
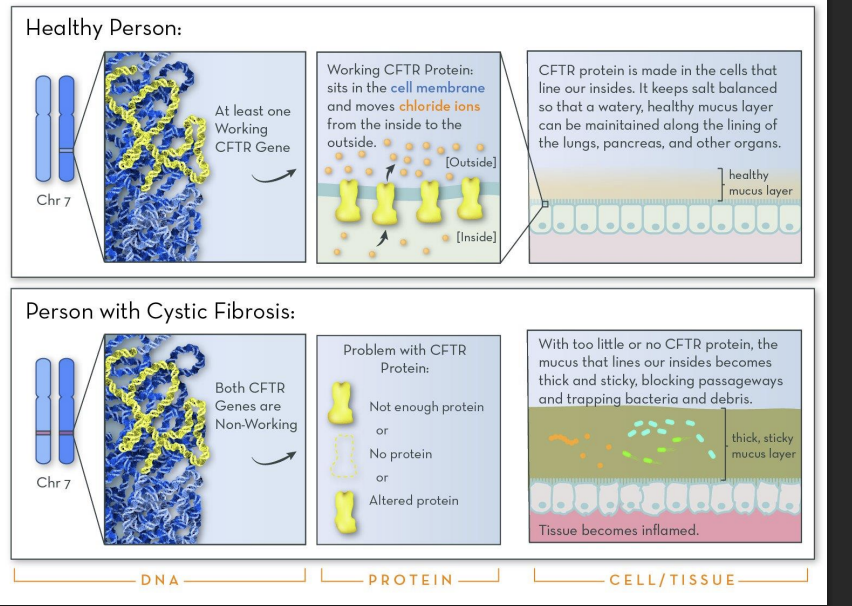
ER Protein Folding Disease: Cystic Fibrosis
In some genetic diseases, a genetic mutation leads to the expression of a mutant protein (cannot fold properly into native form).
This mutant protein is recognized by the quality control pathway in the ER AND IS DESTROYED!
Loss of protein and protein’s function in the cell
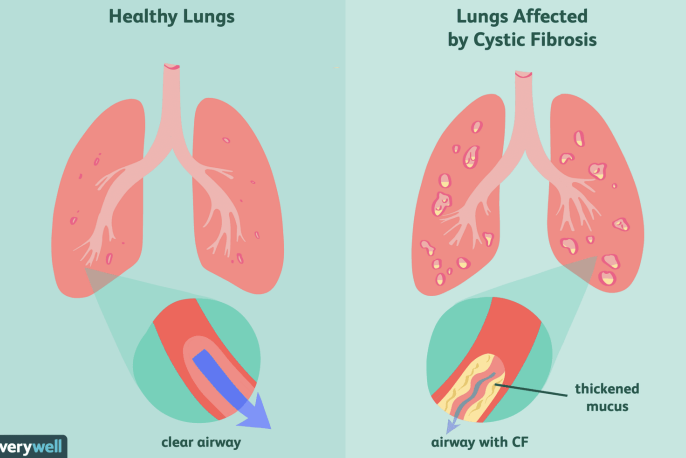
lungs of Cystic Fibrosis
Cystic Fibrosis treatments
How Do Vesicles Transport the Correct Cargo to the Right Place?
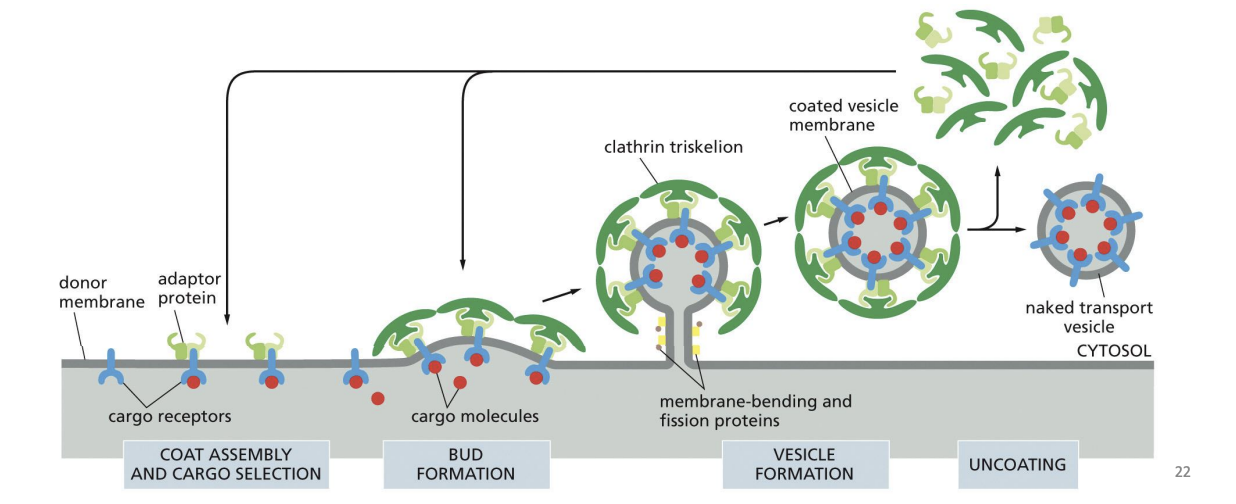
Coat Proteins Mediate Vesicle Cargo and Budding
Coat proteins
Budding vesicles have a “coat” of soluble (cytosolic) proteins that assemble on the membranes of the donor compartment.
they are proteins that mechanically force the membrane to curve and form a budding vesicle
Coat proteins also bind to specific transmembrane receptors that can recruit cargo
Types of Coat Proteins in Vesicle Budding
COP II vesicles
COP I vesicles
Clathrin coated vesicles
COP II vesicles
transport proteins “forward” from ER to the Golgi (anterograde)
COP I vesicles
transport proteins “backwards” from Golgi to ER and within Golgi (retrograde)
Clathrin coated vesicles
Protein sorting to PM, lysosomes (& vacuoles), endocytosis
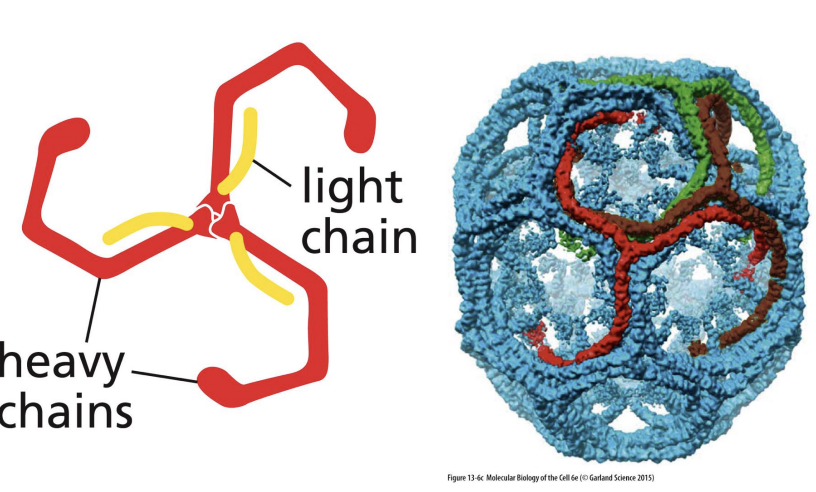
Clathrin
6 proteins (3 heavy chains and 3 light chains) that form “triskelion” structure
Triskelions come together to form a “basket”.
Adaptor Proteins in Clathrin Coats
Several adaptor proteins can bind clathrin and form the “inner coat” between the vesicle membrane and clathrin shell.
Phosphatidylinositol phosphates (PIPs)
Membrane phospholipids (Phosphoinositides or PI) bind to specific adaptor proteins based on the phosphorylation of their inositol sugar at the 3’, 4’ and 5’ position
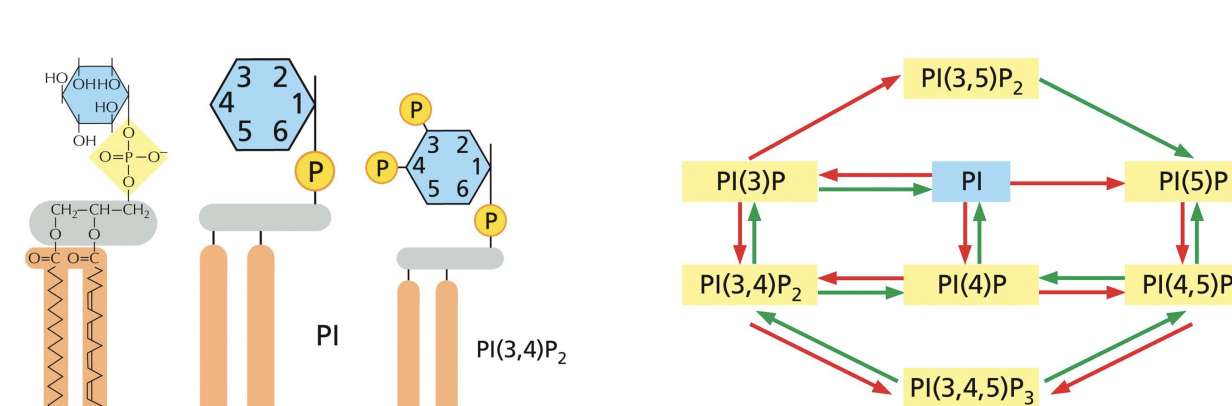
Adaptor Proteins Control Coat Assembly
Different organelles have different phosphoinositidyl phosphates (PIPs) due to different PIP kinases and phosphatases.
These differentially phosphorylated inositol groups can bind to different adaptor proteins.
AP2 adaptor (multi-subunit adaptor protein) binds to PIPs and cargo receptors on cell surface.
Binding triggers internalization of protein complex and assembly of clathrin coated vesicles
The adaptor protein AP2 simultaneously binds to a phosphoinositide head group and to a cargo receptor, greatly enhancing its binding to the plasma membrane.
A. True
B. False
a
how does Vesicle Budding Depends on Membrane Fission
Dimeric membrane-bending proteins → help membrane curvature (bend) at sites where adaptor proteins assemble.
Fission proteins (e.g., dynamin) → responsible for cutting the vesicle free (severing of vesicle).
Dynamin is a GTP-binding protein that assembles into a collar around the neck of the budding vesicle.
GTP hydrolysis by dynamin provides the energy for conformational changes that tighten the collar and breakoff the vesicle from the donor membrane.
curvature → neck formation → dynamin collar → GTP hydrolysis → fission.
how does Targeted Vesicle Transport work
Vesicles have specific proteins that control their movement and fusion to specific target organelle
Targeted Vesicle Transport mediated by
Mediated by three types of proteins:
Coat recruitment GTPases
Rab proteins
SNAREs
Coat recruitment GTPases =
initiate coated vesicle formation.
Rab proteins =
direct vesicles.
SNAREs =
docking and fusion.
COP II coat
several proteins in a large complex
Role of Coat-Recruitment GTPases in Vesicle Transport
1. An ER-membrane GEF recruits Sar1 to the ER and catalyzes the exchange of the bound GDP for GTP.
2. This GDP to GTP switch (inactive to active) causes a change in Sar1 protein structure and it extends through the membrane, causing it to bend.
3. Sar1-GTP recruits other proteins that also increase the bending of the membrane and recruit cargo into the forming COP II coated vesicle.
These other proteins (Sec 23/24) bind cytosolic side of ER transmembrane receptors to recruit cargo proteins.
4. More cytosolic COP II proteins (Sec 13/31) join the complex to form outer structural layer. Once coat is assembled, the vesicle is pinched off
GTP hydrolysis = release of Sar1 and all other COPII proteins.
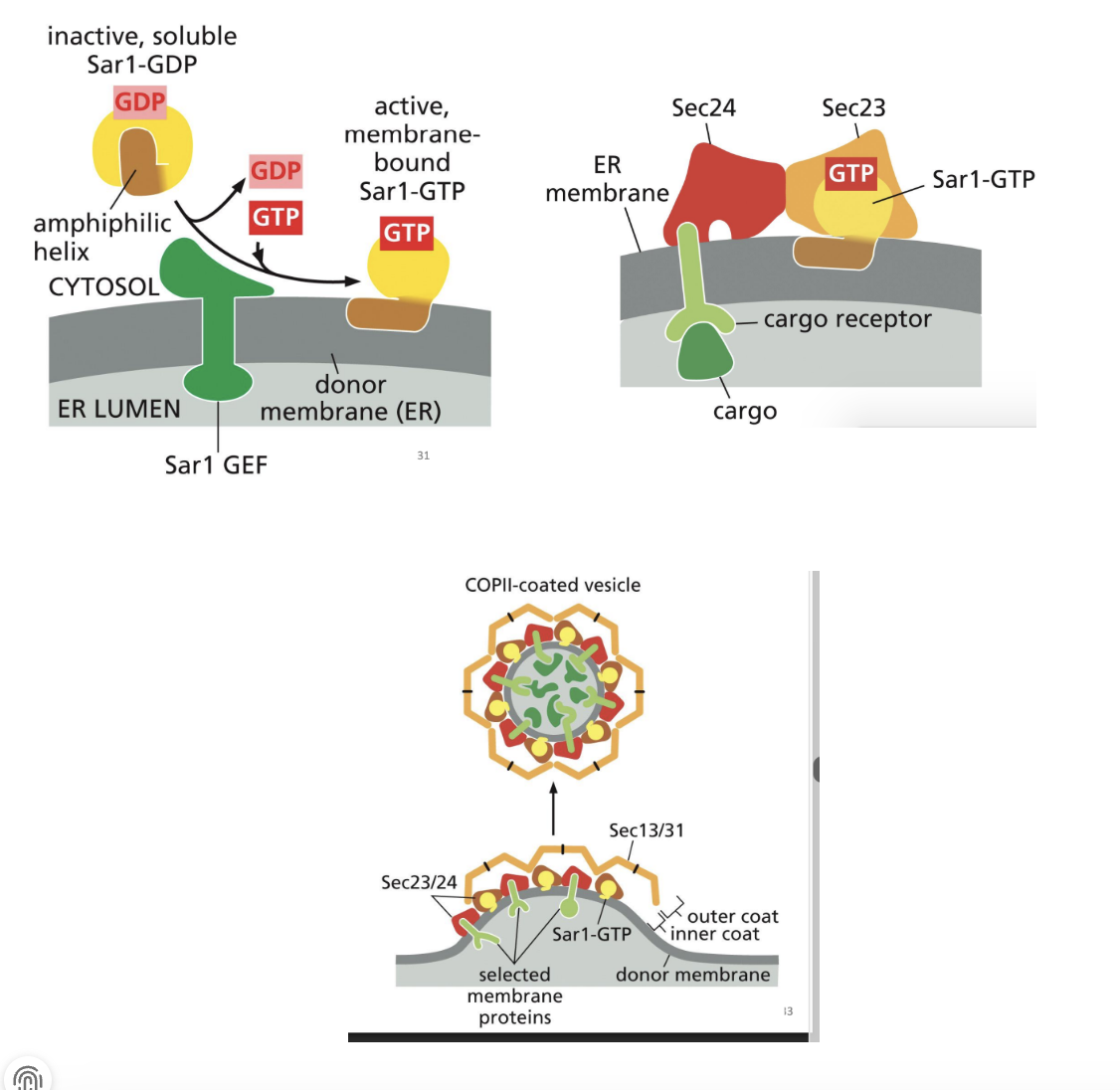
Model of Proteins Moving From ER to Golgi
Exit signals are unknown.
Some cargo receptors bind the sugars on cargo proteins to recruit into COPII vesicle
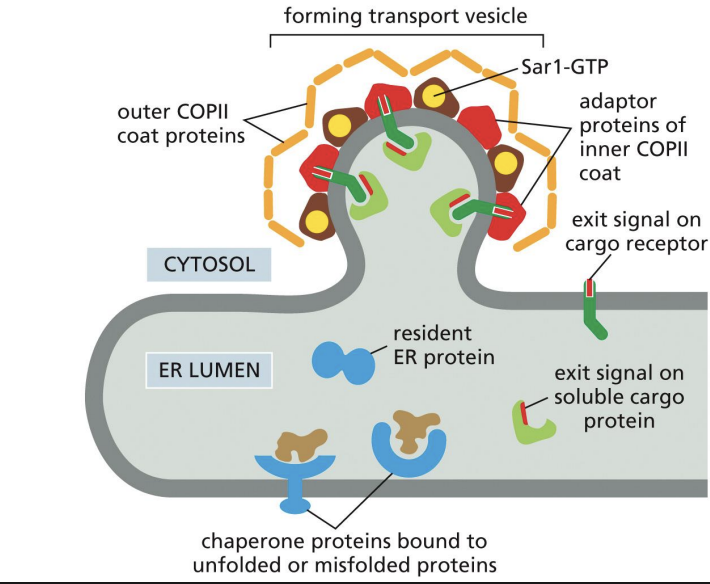
Which of the following proteins is fully or partially exposed on the lumenal surface of COPII vesicles budding from the ER?
A. Sar-1 GTP proteins
B. COPII coat proteins
C. Cargo receptor proteins
D. Adaptor proteins
c
Two types of proteins are involved in targeting vesicles to specific compartments:
• Rab proteins
• SNAREs
Steps involved in in targeting vesicles to specific compartments:
• Tethering (mediated by Rab proteins)
• Docking (mediated SNARE proteins)
• Fusion (mediated by SNARE proteins)
Tethering of vesicles
Extended fibrous proteins and multiprotein tethering complexes “tether” vesicle to target compartment
Rab (Ras-associated binding) proteins role in Vesicle Tethering
small GTP-binding proteins
• Associated to membranes by a lipid anchor
• Specific Rab proteins associate with specific membranes/organelles
• Recruit tethering proteins from the cytosol
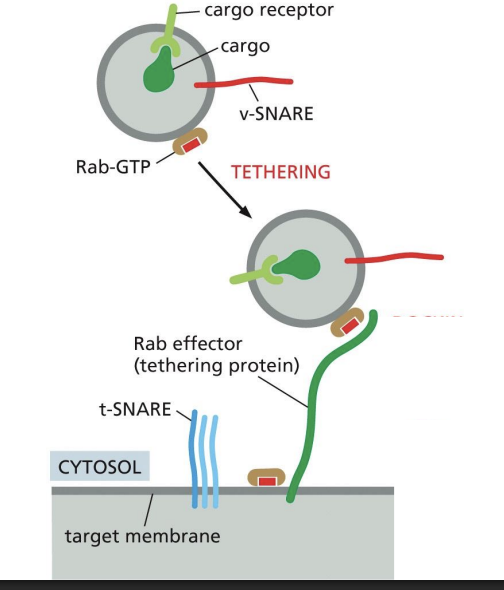
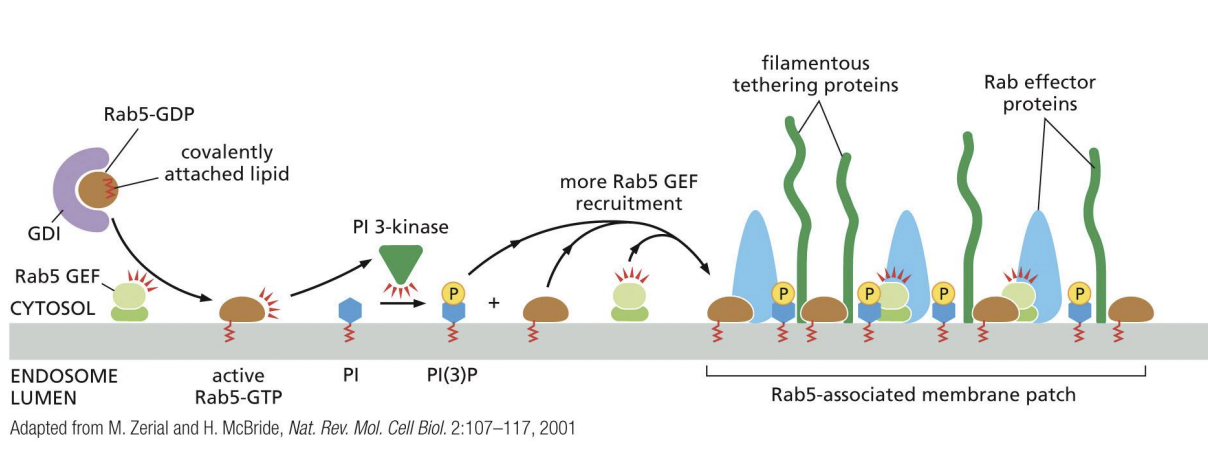
Positive feedback loop of active Rab5
Positive feedback loop of active Rab5 recruiting Rab5 GEFs to generate more active Rab5.
Docking of vesicles
• Process that leads to vesicle fusion
• Requires interaction between membrane proteins on the vesicle and on the target (recipient) membrane
SNARE (Snap Receptor) protein family role in Vesicle Docking
• v-SNAREs on vesicle membrane
• t-SNAREs on target membrane
• Interaction pulls respective membranes very close together
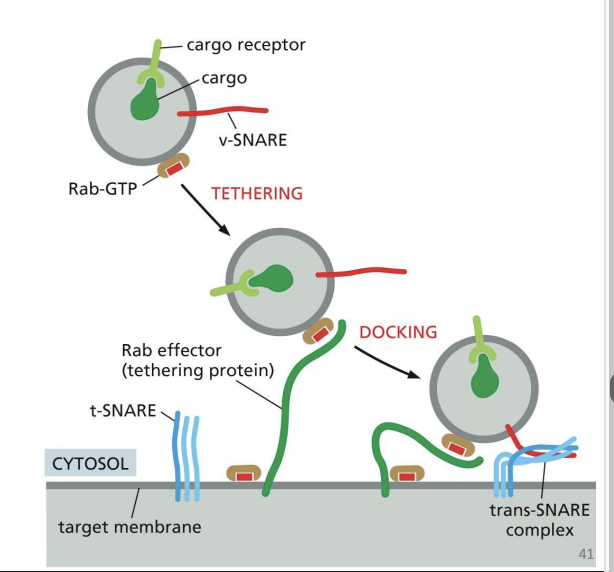
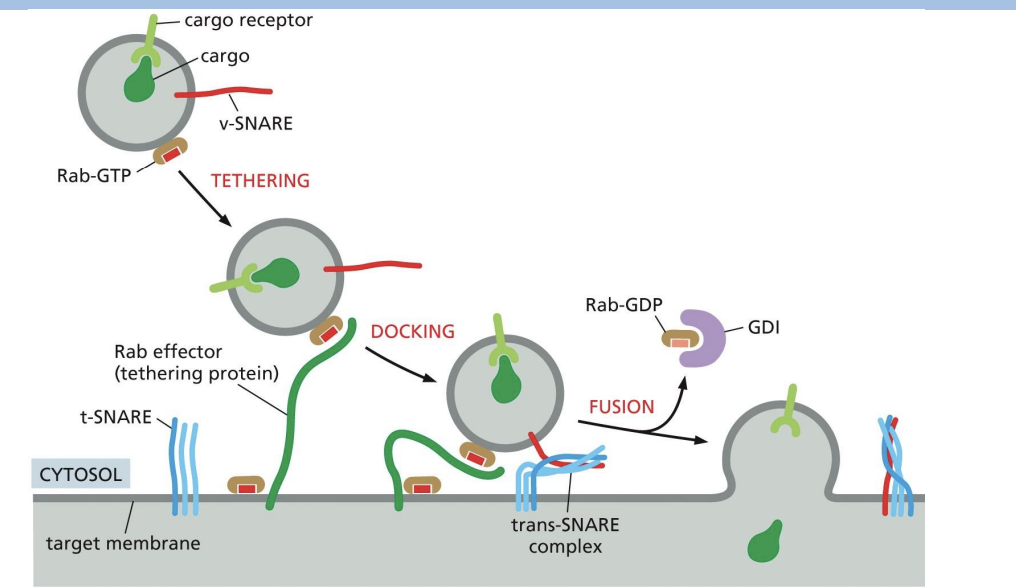
Fusion of vesicles
SNARE proteins allow for the two membranes to create a new bilayer
what happens to snare proteins after fusion
SNARE Proteins Must Be Pried Apart After Fusion
Unzipping of SNARE proteins needs
NSF protein (ATPase).
what happemns to v-SNAREs after fusion
v-SNAREs are retrieved and returned to compartment of origin.
Summary of Vesicle Tethering, Docking and Fusion
Vesicular Tubular Clusters
After COPII coat is lost, ER derived vesicles fuse together
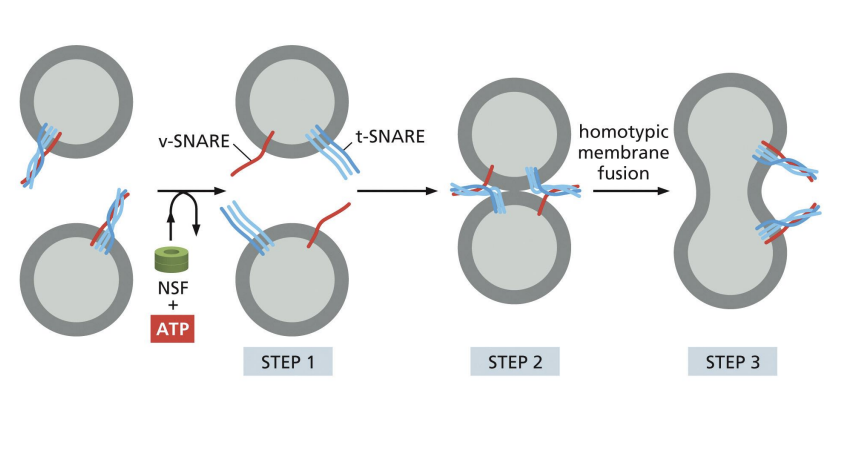
ERGIC
ER-Golgi Intermediate Compartment
ER Protein Retrieval From Golgi
ER resident proteins that leave with vesicles to the Golgi get sorted and returned. COPI coated vesicles mediate retrograde transport of proteins from Golgi to ER
COPI coats are recruited as a preassembled complex = Coatomer
How the Cell Knows Which Proteins to Bring Back
ER resident protein retrieval requires a signal sequence!
where is the retrival signal located
C-terminal of proteins:
Membrane = Lys-Lys-X-X (KKXX)
Soluble = Lys-Asp-Gly-Leu (KDEL)
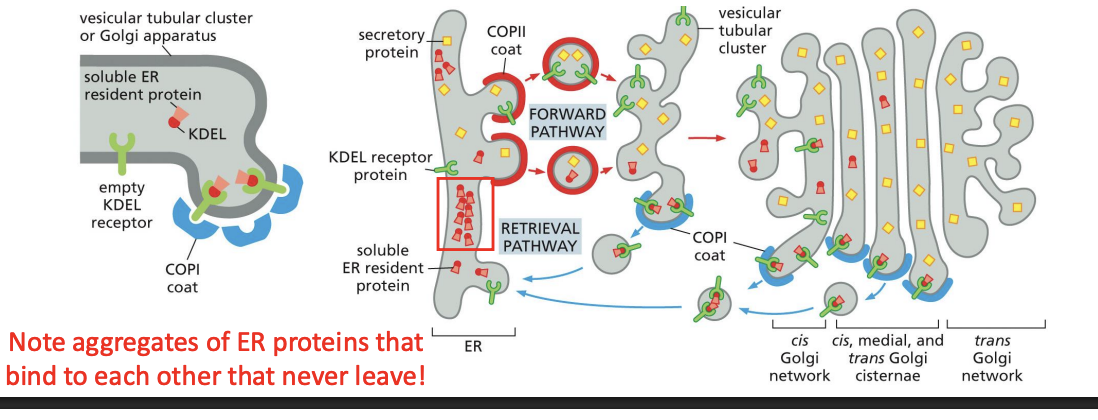
Golgi Apparatus charcateristics (location, strcuture)
“Processing plant” of the cell
• Located adjacent to the nucleus
• Consists of stacks of membranous cisternae (ribbon-like appearance)
• Several functionally distinct compartments (non-uniform from one end to the other)
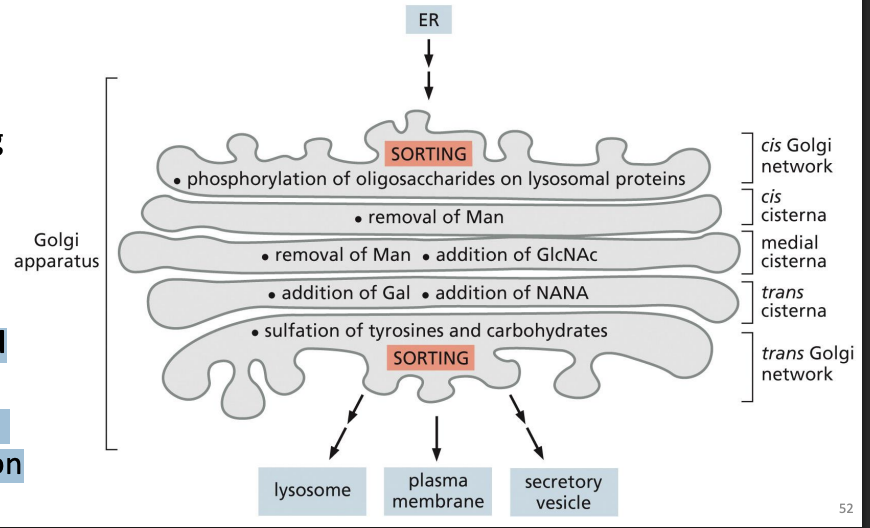
Golgi Apparatus:Progression of proteins
from Cis to Medial to Trans Golgi network with distinct proteins in each stack.
cis-Golgi network (CGN):
A network of fused vesicular tubules coming from ER
Trans-Golgi network (TGN):
A network of tubules and vesicles that sort proteins to final destination.
Golgi Apparatus function
Proteins are processed (e.g. cleavage), modified (e.g. glycans) and sorted = functional compartmentalization
Why are proteins glycosylated?
1) Keeps intermediates of protein folding “soluble” and prevents aggregates.
2) Sequential modification signify progression of protein folding = glyco-code
3) Can act as protective coat
3) Can modulate protein function
Golgi: Protein Modification
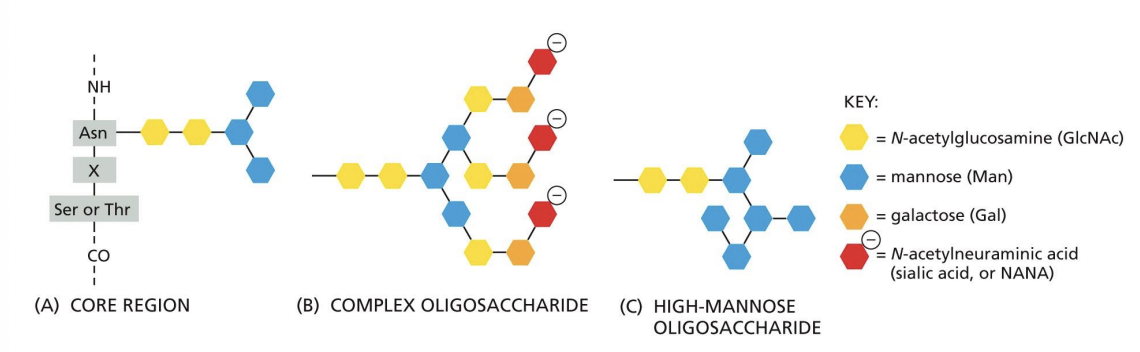
Complex Oligosaccharides or Complex N-glycosylation = N-glycosylation oligosaccharide sugars are trimmed, and other sugars are added.
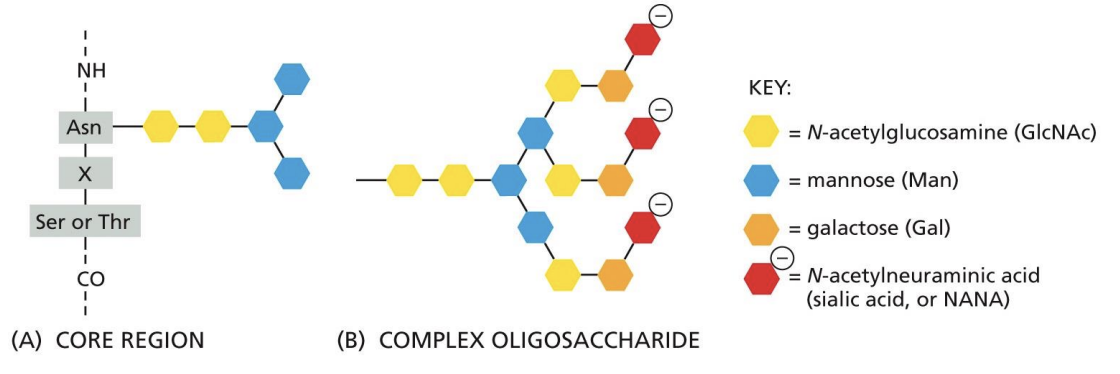
High Mannose Oligosaccharides
N-glycosylation oligosaccharide sugars are trimmed, and NO sugars are added.
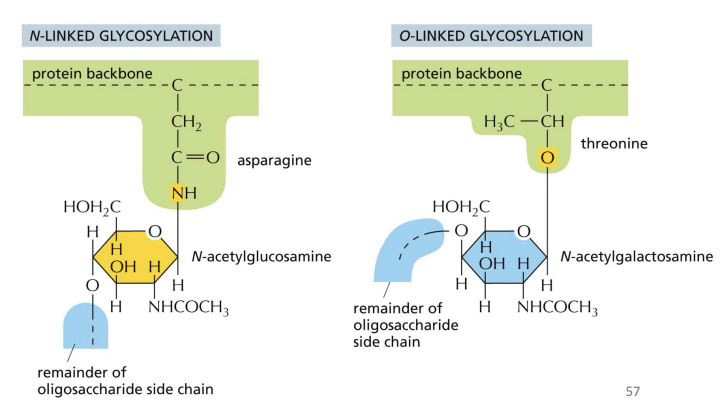
O-linked glycosylation
another form of modification where sugars are attached to serine or threonine on specific proteins.
Typically added after protein folding, sequence directing addition is unknown.
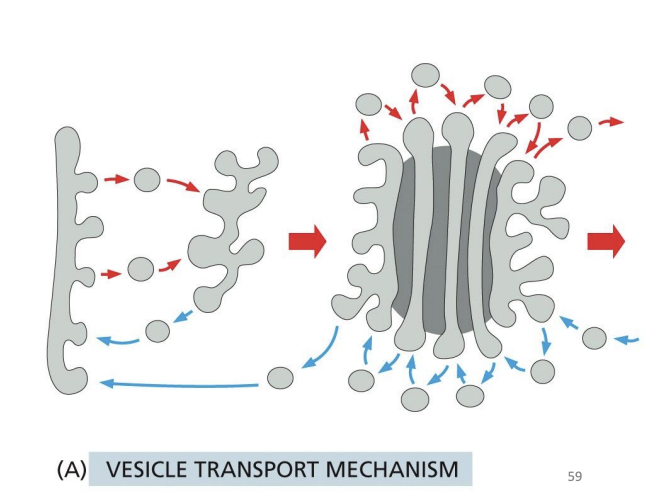
How Does the Golgi Maintain Polarized Structure?
Hypothesis 1: Vesicle Transport Mechanism
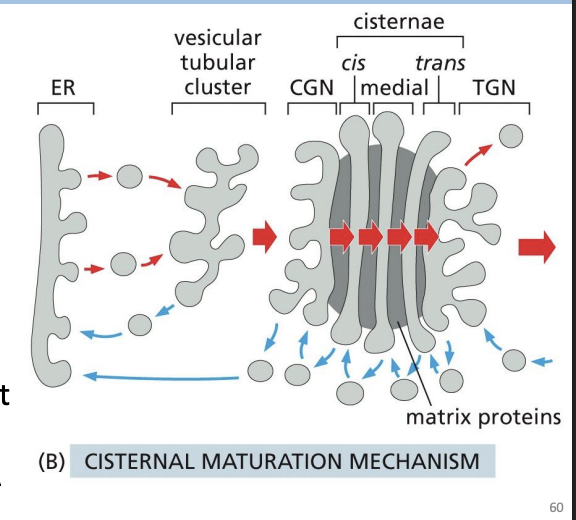
Hypothesis 2: Cisternal Maturation Mechanism
Hypothesis 1: Vesicle Transport Mechanism
• The stacks are stable structures
• Vesicles bud from one compartment and fuse with the next one
• Move non-resident proteins forward
• Changes the composition of each of the cisternae
Hypothesis 2: Cisternal Maturation Mechanism
• Cisterna formed by transport vesicles that originate in the ER
• Cisterna physically move from the cisend to the trans-end (change their composition as they move or “mature”)
• Resident proteins are retrieved back from the stacks - retrograde movement
Golgins
cytosolic Golgi proteins that form long tethers to capture transport vesicles by interacting with Rab proteins
Phosphorylated Golgins dissociate
golgi fragmentation and dispersal in cytosol.
Important for mitosis and segregation of Golgi in both daughter cells!
Lysosomes
digestive organelles
Considered “bags” of hydrolytic enzymes that are typically acid hydrolases (work in acidic pHs).
Break down macromolecules and move products (e.g. amino acids, nucleotides) back to cytosol.
Vacuolar H+ ATPases
drive H+ into lumen (low pH) using ATP
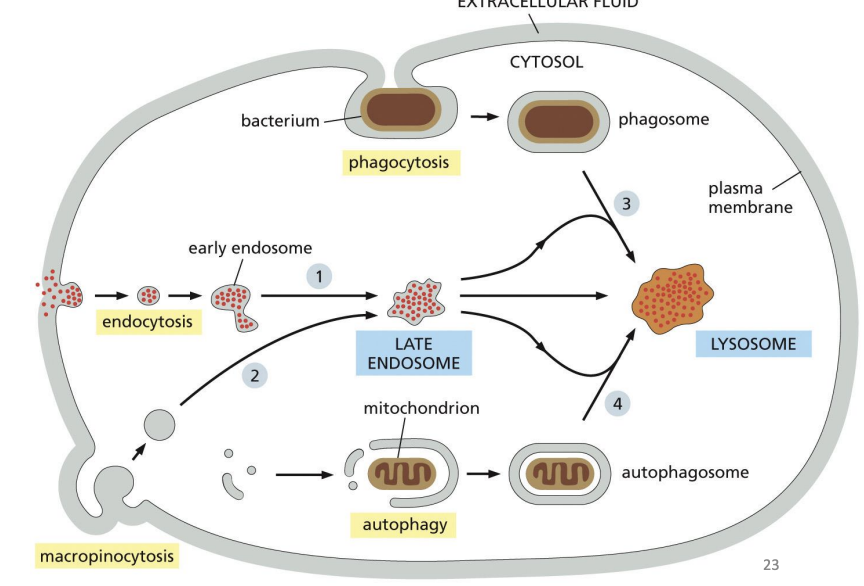
4 major pathways of delivery to Lysosomes
Phagocytosis
Endocytosis
Autophagy
Macropinocytosis
Secretion of Proteins from Golgi
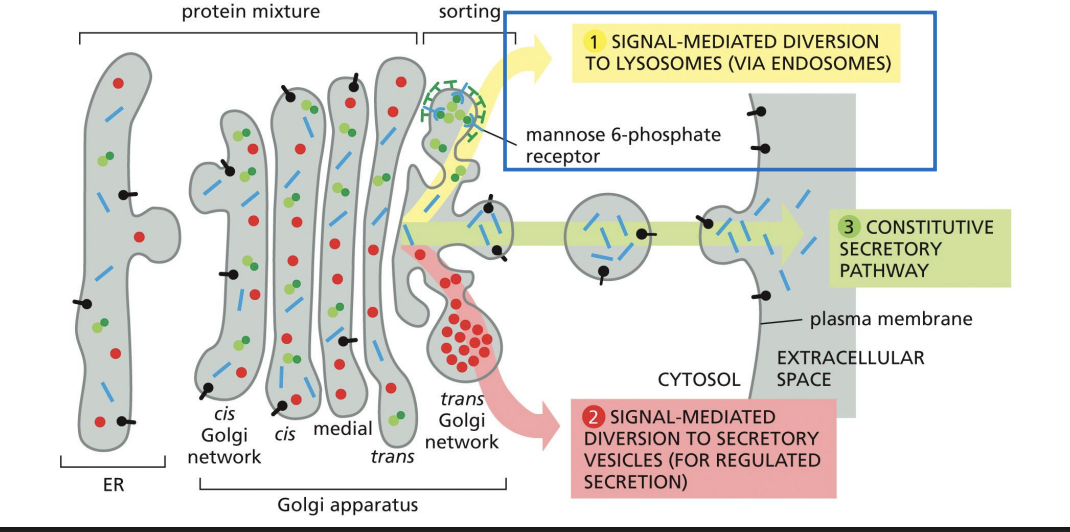
Protein Sorting: Lysosomal Enzymes
1) Lysosomal enzymes are synthesized, Nglycosylated and folded in ER
2) Transported to Golgi by COPII vesicles
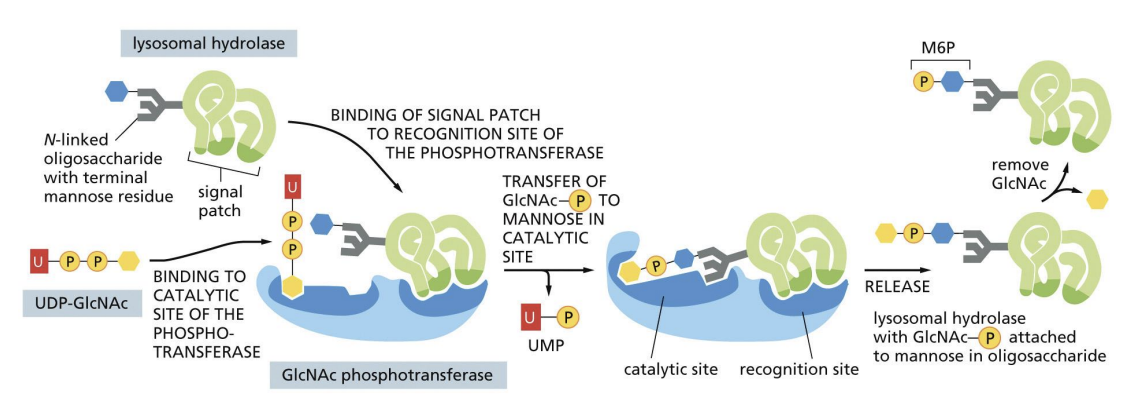
3) Specific mannose sugars of N-linked glycans are phosphorylated in Cis Golgi
lysosomal enzyme targeting via the Mannose-6-Phosphate (M6P) pathway
Mannose phosphorylation = signal for lysosome transport.
Cluster of amino acids = signal patch that allows for M6P signal to be added.
Mannose phosphorylated proteins bind receptors (MPRs) in TGN and are recruited into Clathrin coated vesicles.
Vesicles fuse with endosomes that become lysosomes.
MPRs are recycled back to Golgi.
Protein Sorting: Vacuolar Enzymes
Vacuoles are like lysosomes in plant cells!
Vacuolar proteins have vacuolar targeting sequence (VTS).
VTS is recognized by sorting proteins in TGN and are targeted to vacuole in vesicles.
VTS is cleaved off upon arrival = mature protein
Lysosomal Storage Disorders
Enzymes are not delivered to lysosomes which results in diseases where there is a buildup of material in lysosomes
I-cell disease
Patients have a mutation in a gene that codes for a protein involved in mannose phosphorylation of proteins
• No M6P = no signal for transport to lysosomes
• Lysosomal enzymes are transported to the cell exterior instead = constitutive secretion
Material to be degraded accumulates within swollen lysosomes in cell.
Patients have severe skeletal and developmental abnormalities.
Exocytosis
process by which the contents of secretory vesicles are released into the extracellular environment
Vesicle lumen becomes part of the extracellular space
• Soluble proteins are released
• Membrane proteins become inserted into the PM (glycosylation sites face outside of cell)
Constitutive secretion (default pathway):
Synthesis and secretion of proteins continuously, in an unregulated manner.
• Most membrane proteins
• Most proteins that make up the extracellular matrix (ECM)
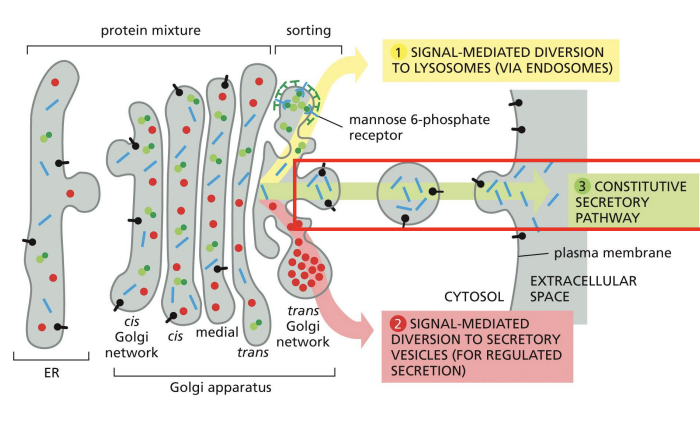
Regulated secretion:
Proteins to be secreted are densely packaged into large vesicles (secretory granules) that wait for a cellular signal before they release their contents.
Ex. hormones, neurotransmitters, digestive enzymes
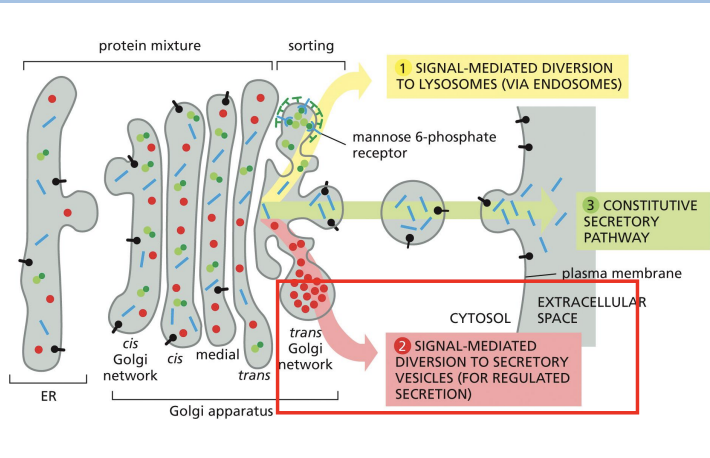
Secretion of Proteins from Golgi
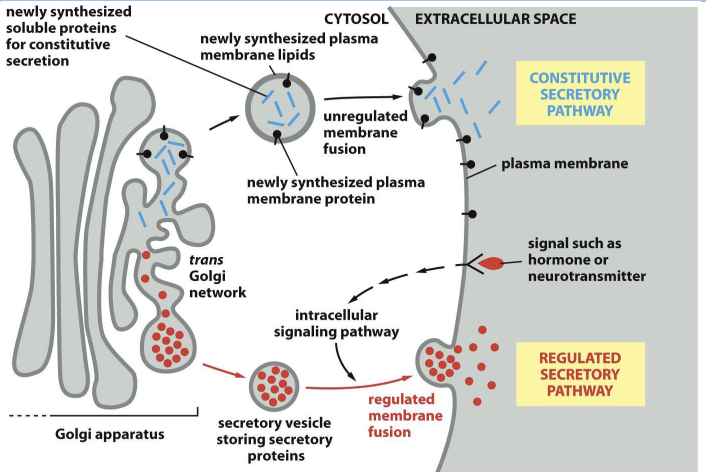
Regulated Secretion of Proteins from Golgi
Proteins to be secreted by regulated secretion seem to aggregate for entry into secretory vesicles by an unknown mechanism
Calcium ion concentration can trigger regulated secretion.
Synaptic vesicles
specialized class of small secretory vesicles.
Synaptic vesicles dock at the plasma membrane and undergo a priming step to prepare for rapid fusion.
Synaptic Vesicle Fusion With Plasma Membrane
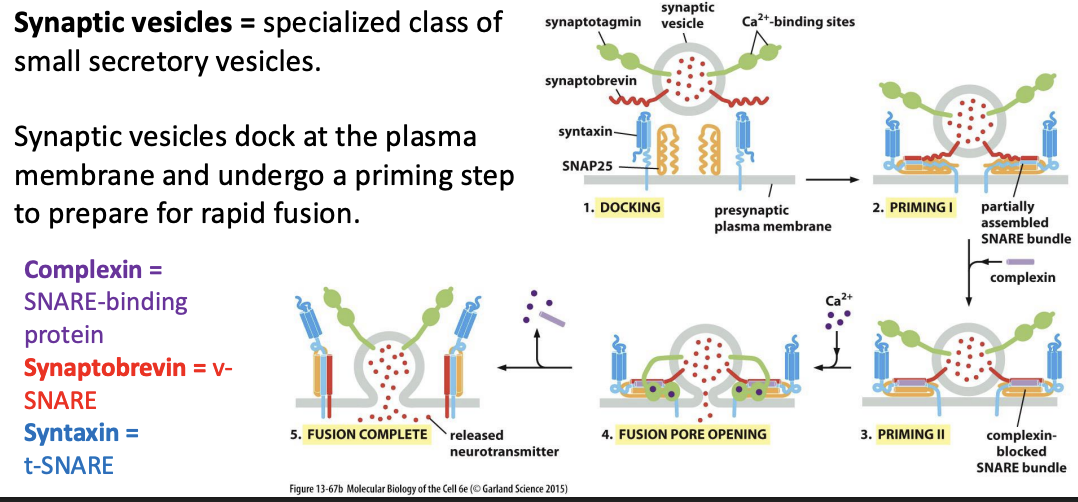
Imagine that the Sar1 protein was mutated so that it could not hydrolyze GTP, regardless of its binding partners. When bound to GTP upon interaction with a Sar1 GEF on the ER membrane, would the mutant Sar1 direct proper assembly of COPII-coated vesicles? Would these COPII coats, if formed, then disassemble normally?
A. COPII coats would not assemble or disassemble properly.
B. COPII coats would assemble and disassemble normally.
C. COPII coats would assemble properly but not disassemble.
D. COPII coats would not assemble but would disassemble.
c
The KDEL receptor must shuttle back and forth between the ER and the Golgi apparatus to accomplish its task of ensuring that soluble ER proteins are retained in the ER lumen. Which compartment—ER or Golgi—would you expect to have the highest concentration of KDEL receptor? Would you expect the KDEL receptor to have a standard ER retrieval signal?
A. The KDEL receptor will be more concentrated in the Golgi; it will not have a standard ER retrieval signal.
B. The KDEL receptor will be more concentrated in the ER; it will not have a standard ER retrieval signal.
C. The KDEL receptor will be more concentrated in the ER; it will have a standard ER retrieval signal.
D. The KDEL receptor will be more concentrated in the Golgi; it will have a standard ER retrieval signal.
a
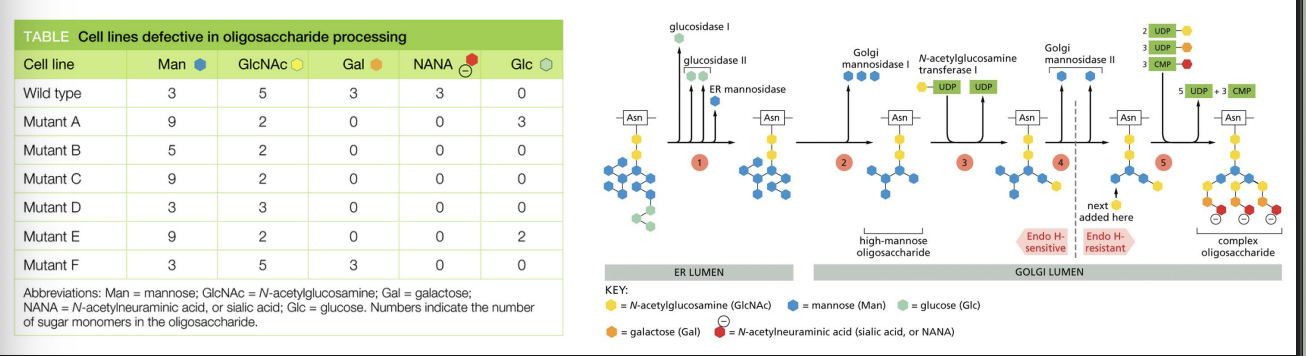
You have isolated several mutant cell lines that are defective in their ability to add sugars to exported proteins. Using an easily purified protein that carries only N-linked complex oligosaccharides, you have analyzed the sugar monomers that are added in the different mutant cells. Each mutant is unique in the kinds and numbers of different sugars contained in its Nlinked oligosaccharides. The pathway for processing complex N-linked oligosaccharides is shown in the figure, with processing steps indicated by numbers in the colored circles
In what order do the enzymes that are defective in mutants B, D, and F normally operate? Mutant B is defective for enzyme B, mutant D is defective for enzyme D, and mutant F is defective for enzyme F.
A) B, D, F
B) B, F, D
C) D, B, F
D) D, F, B
E) F, B, D
F) F, D, B
a
Which statement is TRUE?
A. Constitutive secretion involves secretory vesicles, whereas regulated
secretion does not.
B. It is not possible to have both regulated and constitutive secretion in the
same cell.
C. Only some cells perform regulated secretion while all cells perform
constitutive secretion.
D. Only some cells perform constitutive secretion while all cells perform
regulated secretion.
E. All cells perform both regulated and constitutive secretion.
c
Endocytosis
Process of bringing material from the plasma membrane (or extracellular environment) inside the cell.
Once material enters, there is bi-directional trafficking regulating the fate and final destination.
where does Endocytosis take place
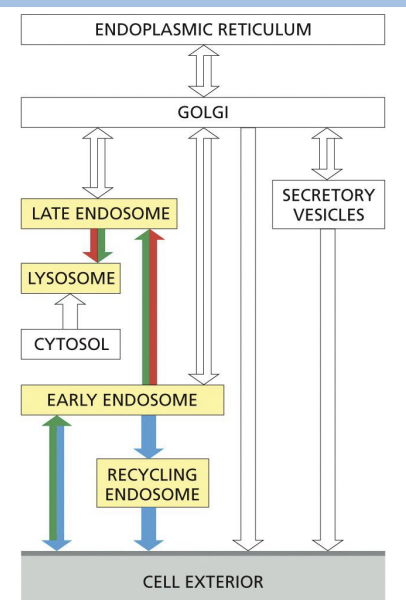
Early endosome
where internalized cargo is sorted.
Recycling endosome =
direct return to PM or storage of proteins for (indirect) PM.
Late endosomes =
mature endosomes that do not send vesicles to PM and can fuse with lysosomes/ endolysosomes.
Which one of the following statements about the control of membrane traffic is correct?
A. The outward flow exceeds the inward flow to maintain sizes of compartments.
B. The outward flow of membrane is less than the inward flow in growing cells.
C. Synaptic vesicles fuse faster than they can recycle, growing the nerve terminal.
D. The outward and inward membrane flows are balanced in a nongrowing cell.
d
Pinocytosis
Process of cell “drinking” = constant formation of endosomes from plasma membrane
Cell surface area and volume doesn’t change!
Mainly clathrin coated vesicles but also get caveolae (cause flask-like invaginations of membranes)
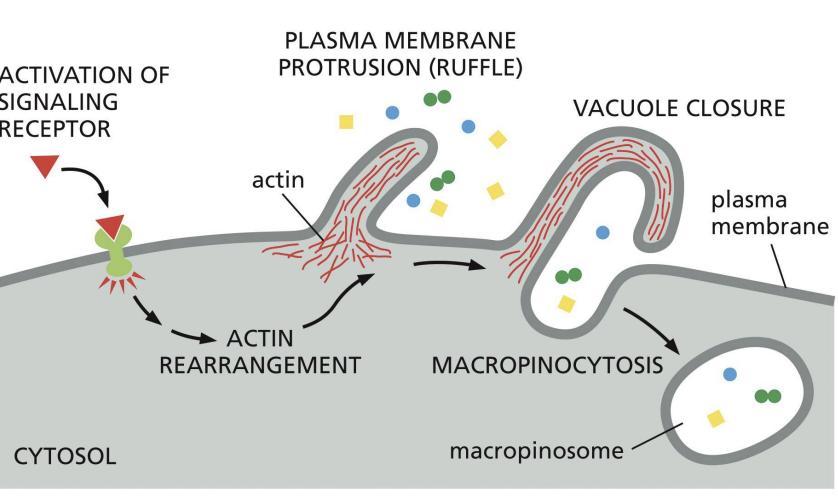
Macropinocytosis
clathrin independent route of endocytosis.
Triggered by signalling pathways that cause PM to ruffle.
Macropinosomes fuse with endosomes = degradation.
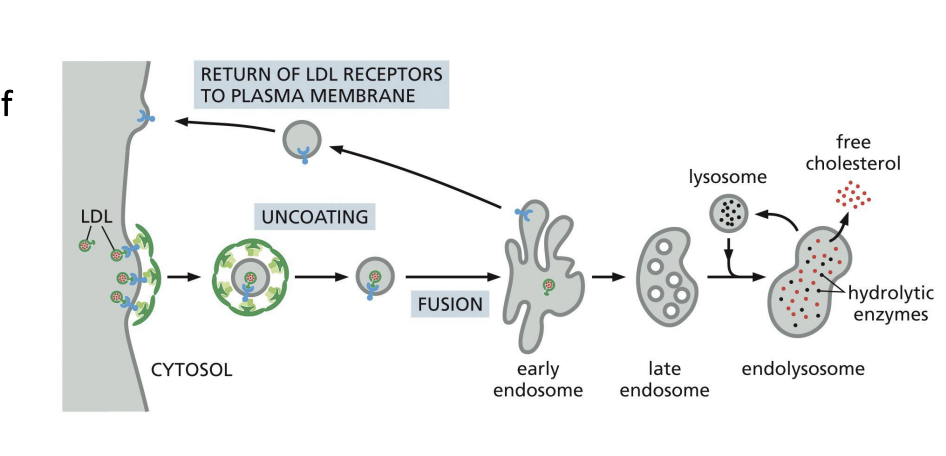
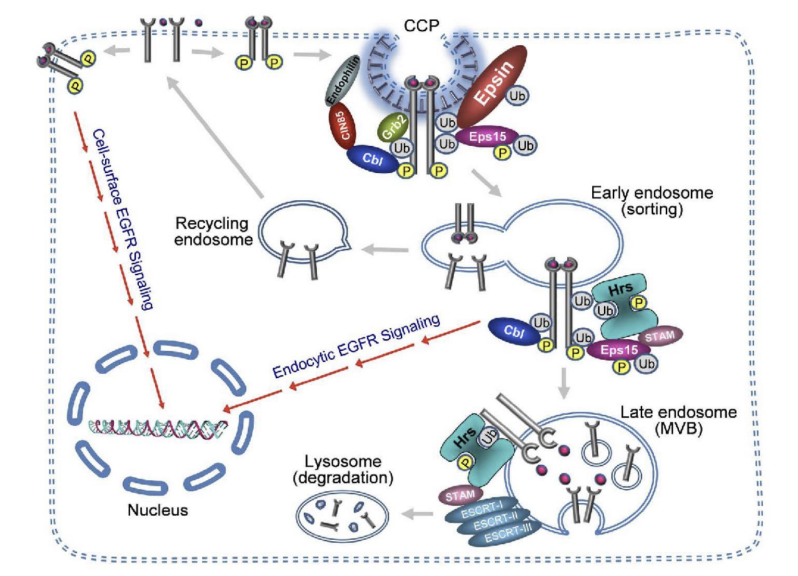
Receptor-mediated endocytosis
cell surface receptors bind specific cargo for internalization e.g. Cholesterol uptake (cholesterol is transported in low-density lipoprotein complexes (LDLs).
When cells require cholesterol, they increase the number of LDL-receptors on PM.
Receptor-mediated endocytosis of LDL allows cell to access cholesterol once the LDL particle is processed in lysosomes
Receptor-Mediated Endocytosis Cargo receptor fates:
1) Recycled back to PM (e.g. Transferrin receptors)
2) Follow the fate of endocytosed material moving to lysosome for degradation (e.g. EGF receptor)
3) Transcytosis (recycled to different PM domain)
1) Recycled back to PM (e.g. Transferrin receptors)
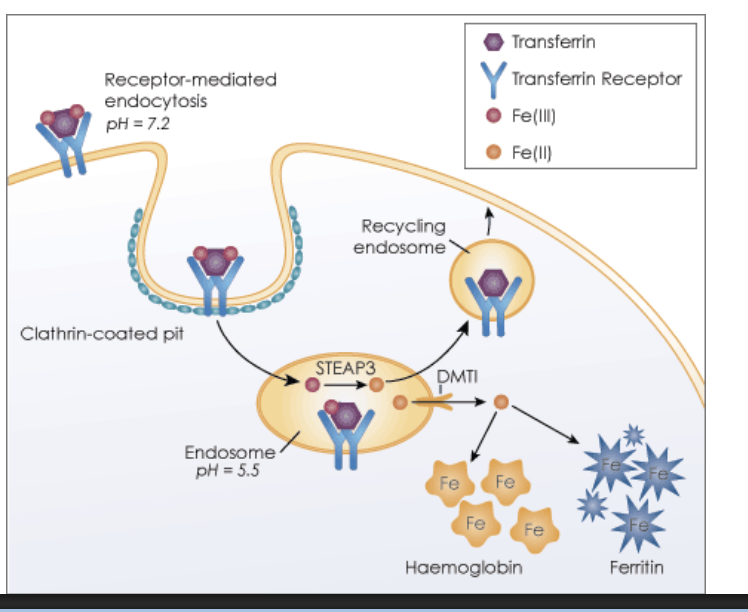
2) Follow the fate of endocytosed material moving to lysosome for degradation (e.g. EGF receptor)
3) Transcytosis (recycled to different PM domain)
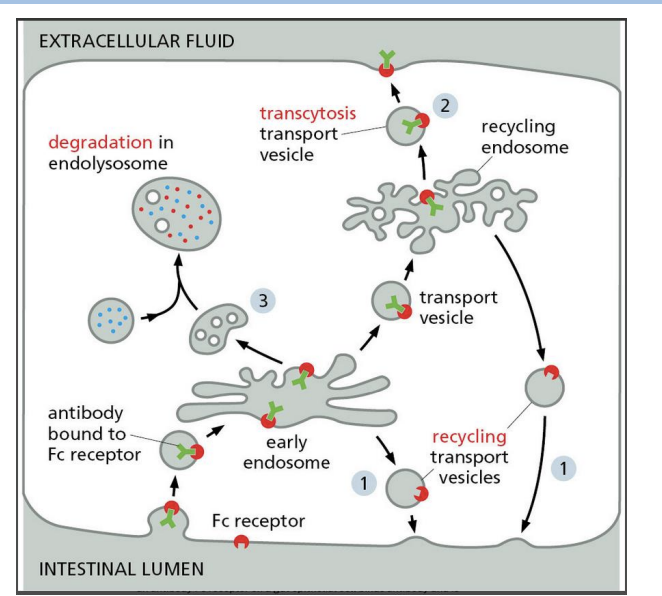
Recycling endosomes in Receptor-Mediated Endocytosis
Recycling endosomes can store proteins and move them to the PM when needed.
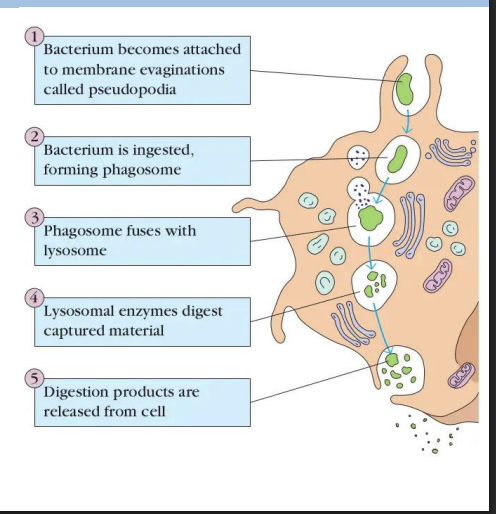
Phagocytosis
form of endocytosis performed by specialized cells (e.g. macrophages) = cell “eating”.
Receptor-mediated internalization that forms large endocytic vesicles (phagosomes).
Some bacteria are able to secrete proteins that prevent fusion of phagosome with lysosome
They live and multiple in these structures e.g. Mycobacterium tuberculosis
Autophagy
“self-eating” = process of cell engulfing obsolete components (e.g. proteins, organelles) in autophagosome to get rid of them.
Nonselective autophagy =
a bulk portion of cytoplasm is sequestered in autophagosomes.
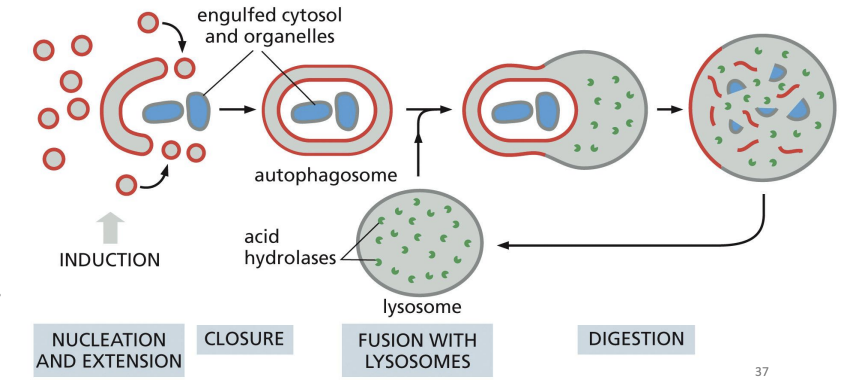
Selective autophagy
autophagosomes tightly enclose specific cargo and mostly exclude the surrounding cytosol.
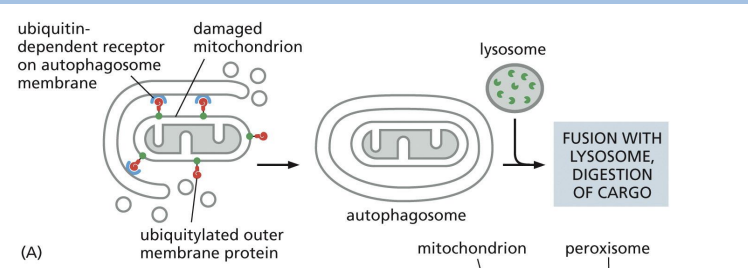
Which of the following is NOT a difference between phagocytosis and autophagy?
A. Only one of the two processes is a type of endocytosis.
B. Autophagosomes and phagosomes have distinct destinations in the cell.
C. Phagocytosis is specific to a few cell types, while autophagy is a common
cellular degradation mechanism.
D. Autophagy degrades intracellular material, while phagocytosis degrades
extracellular material.
b
Plasma membrane has dual function:
• Keep contents of cell in it
• Allow exchange of materials in and out of the cell
Diffusion
substance moves from regions of high to low concentration.
Factors that influence: Diffusion
1) Polarity (more lipid soluble = faster)
2) Size (small uncharged molecules = faster)