Aufbau Principle
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 4:49 PM on 10/22/24
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
Electron Configuration
the arrangement of electrons in their orbitals around the nucleus of an atom.
2
New cards
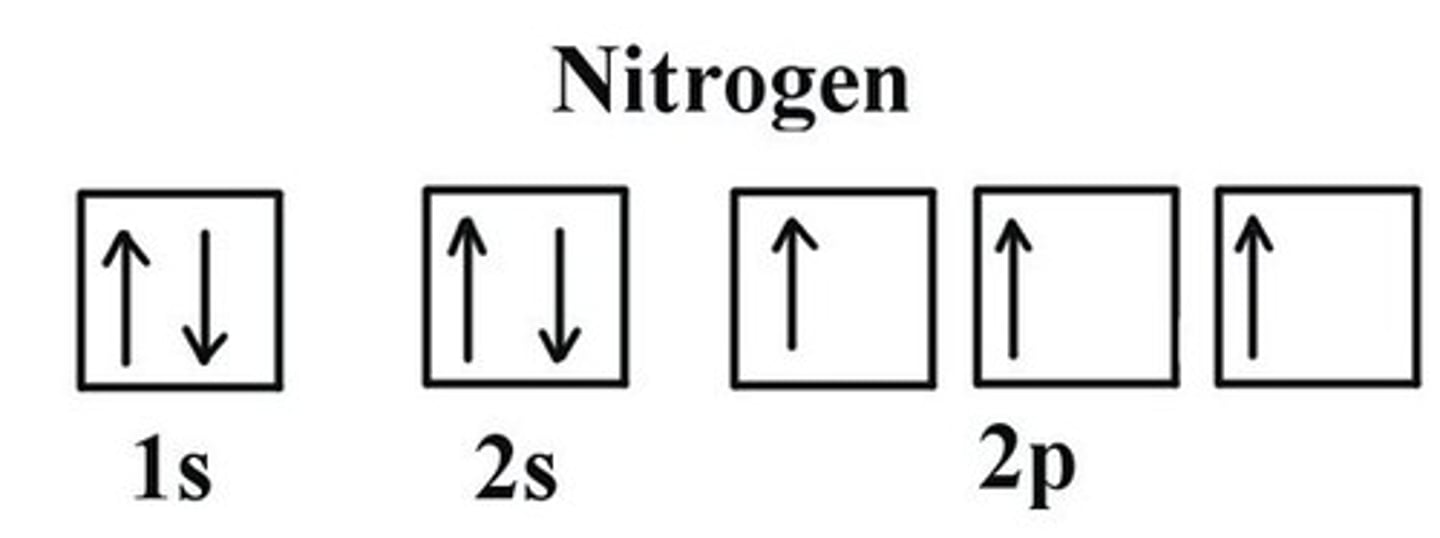
The Aufbau Principle
states tat electrons will always occupy the lowest energy level that is available. (ground state)
3
New cards
The Pauli Exclusion Principle
States that any two electrons in the same atom cannot have the same spin.
4
New cards
Hund's Rule
states that, in orbitals of equal energy, electrons will first occupy different orbitals before pairing up.