CHEM 2300 / Topic 3a: Unit Cells and Close Packing
1/47
Earn XP
Description and Tags
Lecture 19–Lecture 21.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
Why are simple solids so stable?
A solid is a physically condensed state of matter, with additional interactions hold the molecules together.
In simple crystalline solids, such
Ionic bonding
Chemical bonding wherein ions of different elements are held together in inflexible, symmetrical arrays because of electrostatic attractions between opposite charges.
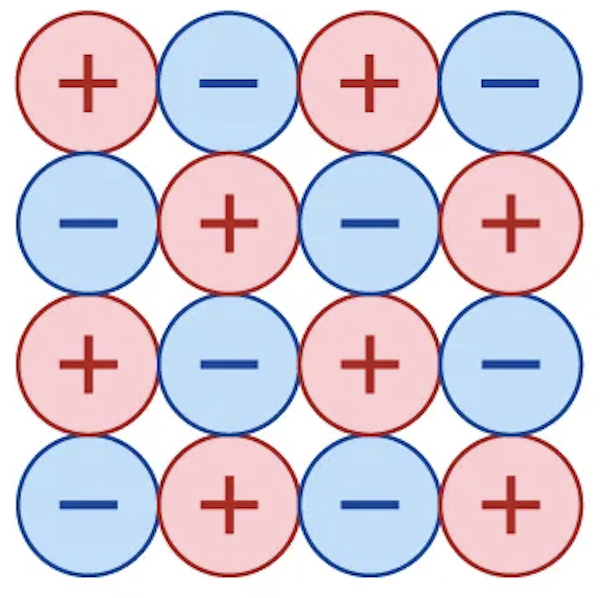
What causes ionic bonding?
Electron transfer, which is present in combinations of elements with large differences in electronegativity.
Metallic bonding
Bonding wherein electrons are delocalized throughout the entire solid.
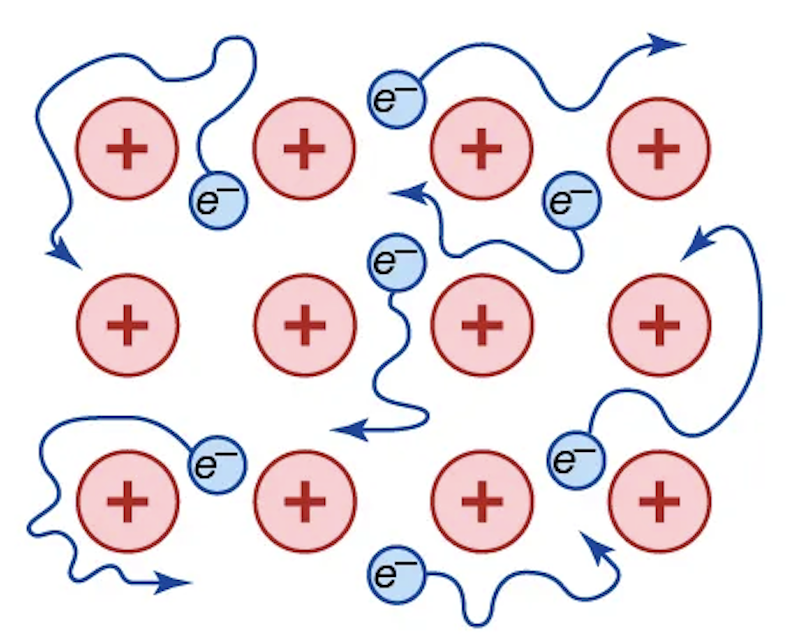
What causes metallic bonding?
Low ionization energies, which is present in elements on the left side of the periodic table, but also extending into the d- and p-blocks.
What is another model of metallic bonding?
One model considers a metal as an enormous molecule with a number of atomic orbitals overlapping to produce a number of molecular orbitals, extending throughout the sample and are very close in energy.
What kind of directionality do metallic and ionic bonds exhibit? What is the reason for this?
No directionality, as structures fill space as efficiently as possible by having no regard for what direction they bond.
Lattice
An infinite three-dimensional arrangement of lattice points, having translational symmetry.
Lattice point
A point having the same structural elements associated with it, the same environment, post-translation.
What are the two types of solids?
Crystalline and amorphous.
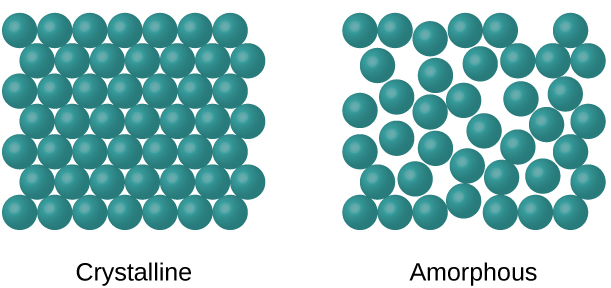
Crystalline solid
A solid with particles arranged in a regular, repeating pattern, having long-range order.
Amorphous solid
A solid with particles not arranged in a regularly repeating pattern, having no long-range order.
Just to be entirely clear, just to nail it in your head, where do a crystal’s macroscopic shape and properties derive from?
Regularly repeating structural elements that make up the crystal.
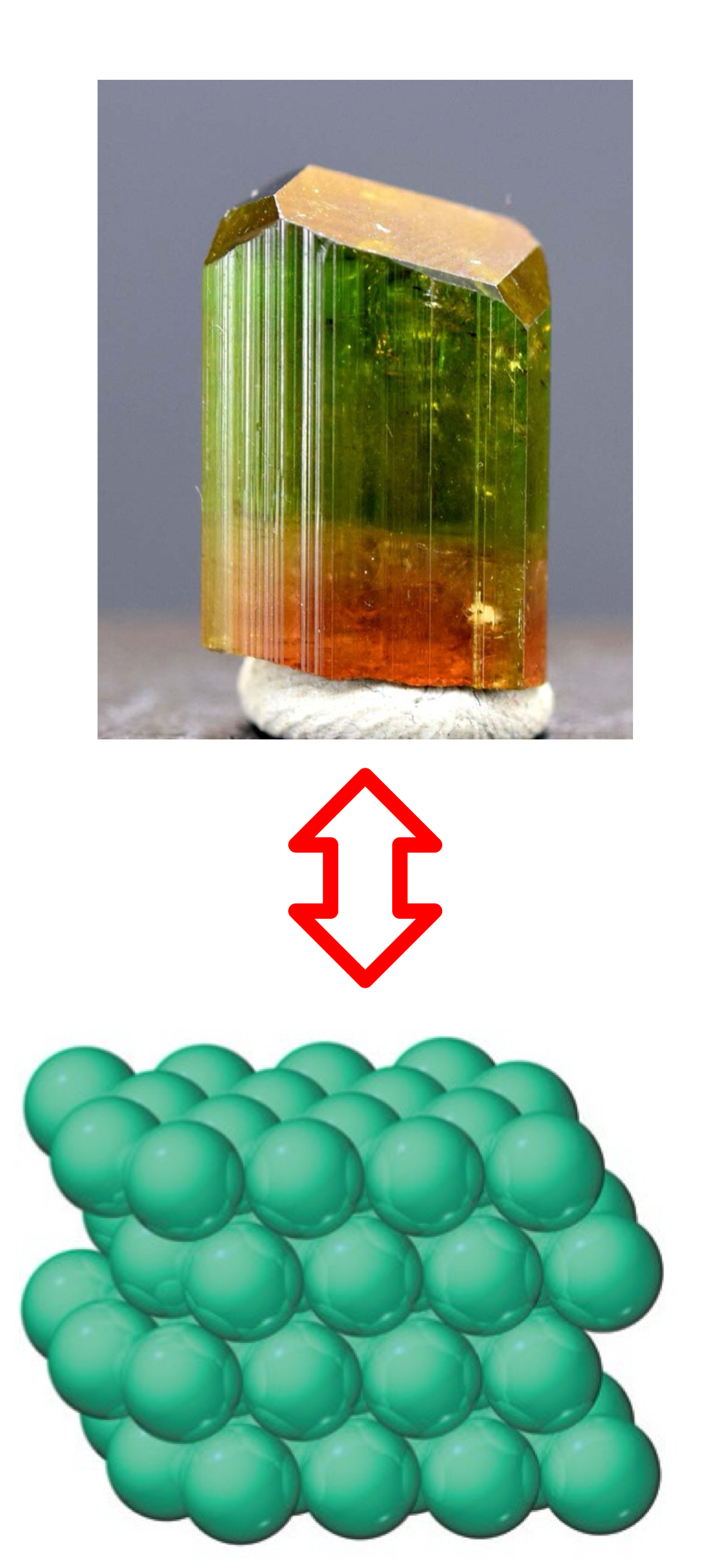
Structural element
An atom, ion, or molecule.
What’s the difference between a crystalline solid and a crystalline lattice?
Crystalline solid is a type of solid, while a crystalline lattice is the arrangement of the particles in the crystalline solid.
Unit cell
The smallest parallel-sided region of structural elements that can be repeated over and over via translational symmetry to reproduce the same three-dimensional array.
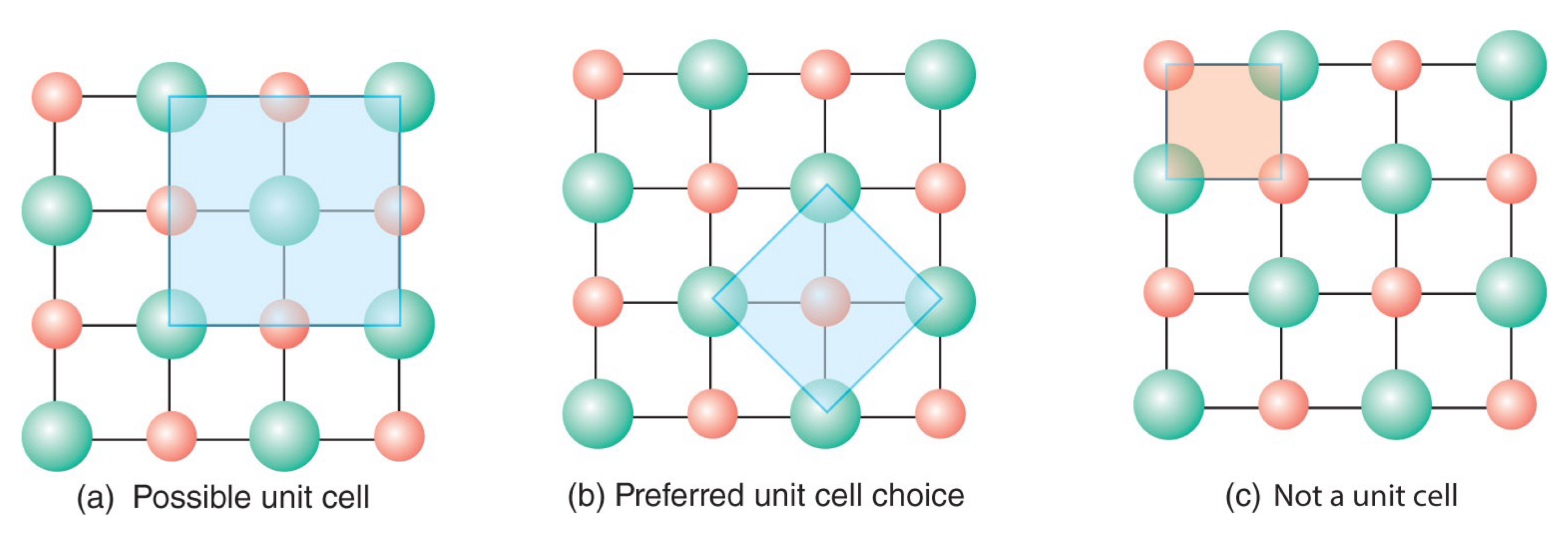
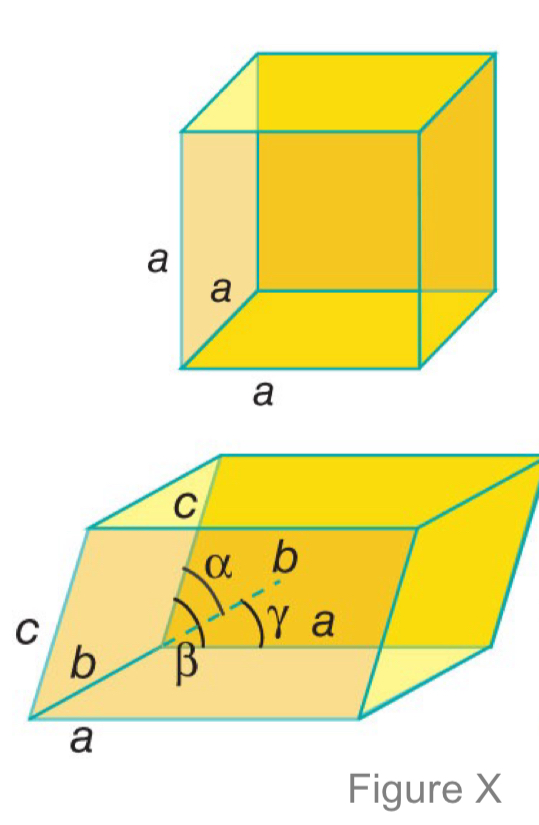
What are the unit cell parameters, a.k.a. the lattice parameters?
The three sides (a, b, and c) and three angles (α, β, and γ). These are properties describing a lattice.
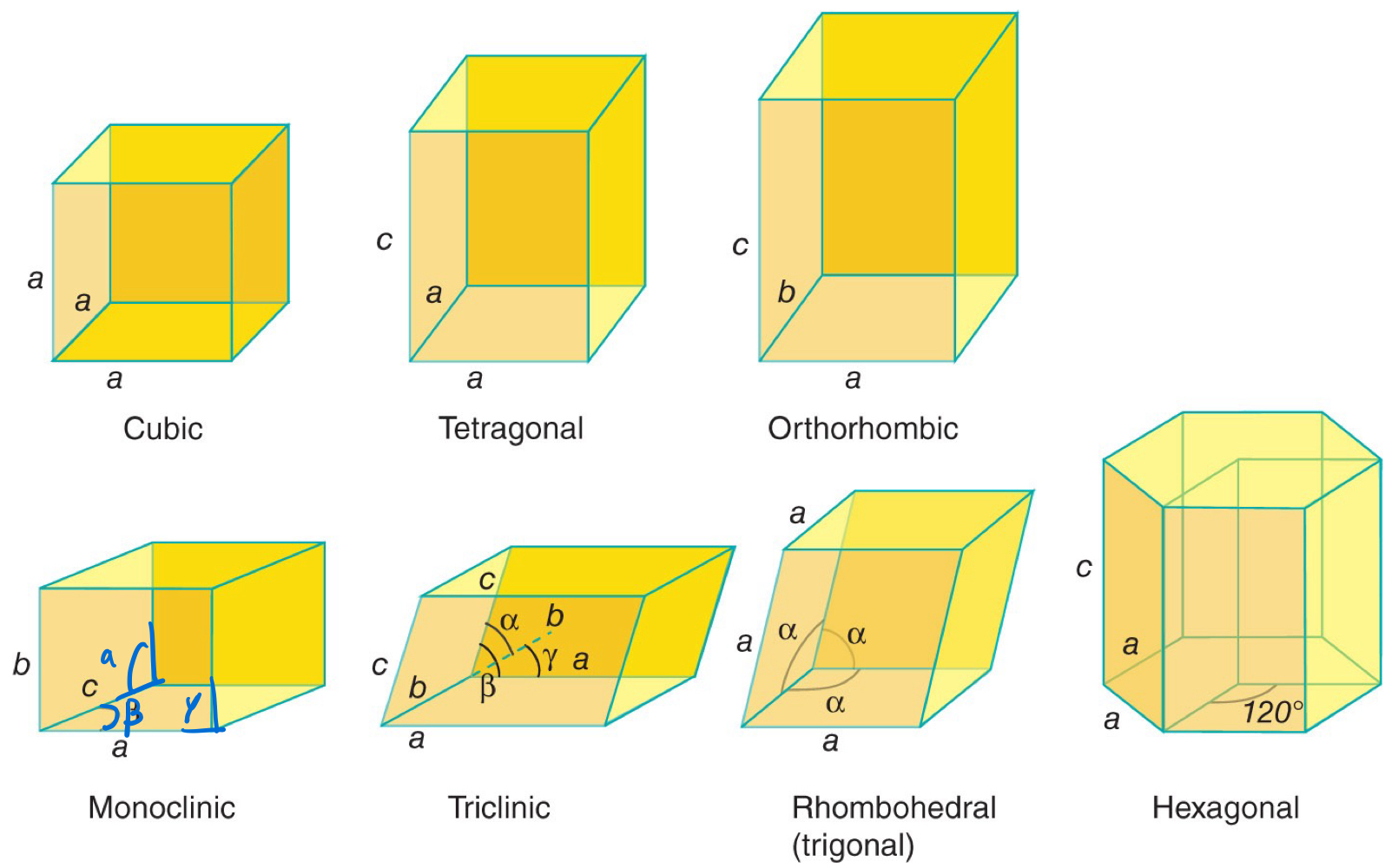
All _____ _______ adopted by compounds belong to one of seven _____ _______.
All ordered structures adopted by compounds belong to one of seven crystal systems.
Primitive unit cell
Cubic unit cell with lattice points only at the corners, containing then in total one lattice point per entire cell.
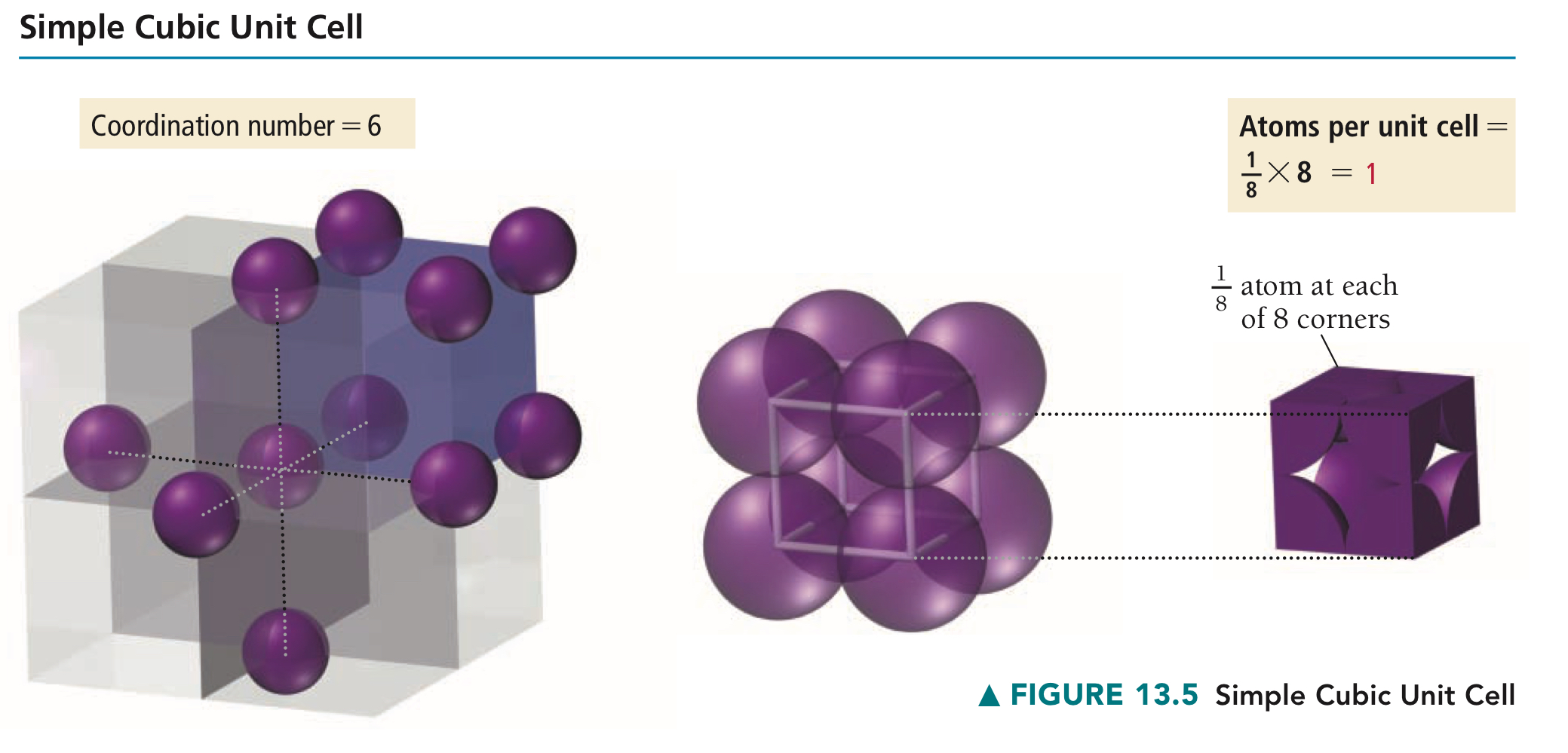
Why does one primitive unit cell have one lattice point in total?
Each lattice point in the corners of a unit cell is actually 1/8 of another unit cell. 8 corners × 1/8 lattice point = 1 lattice point per unit cell.
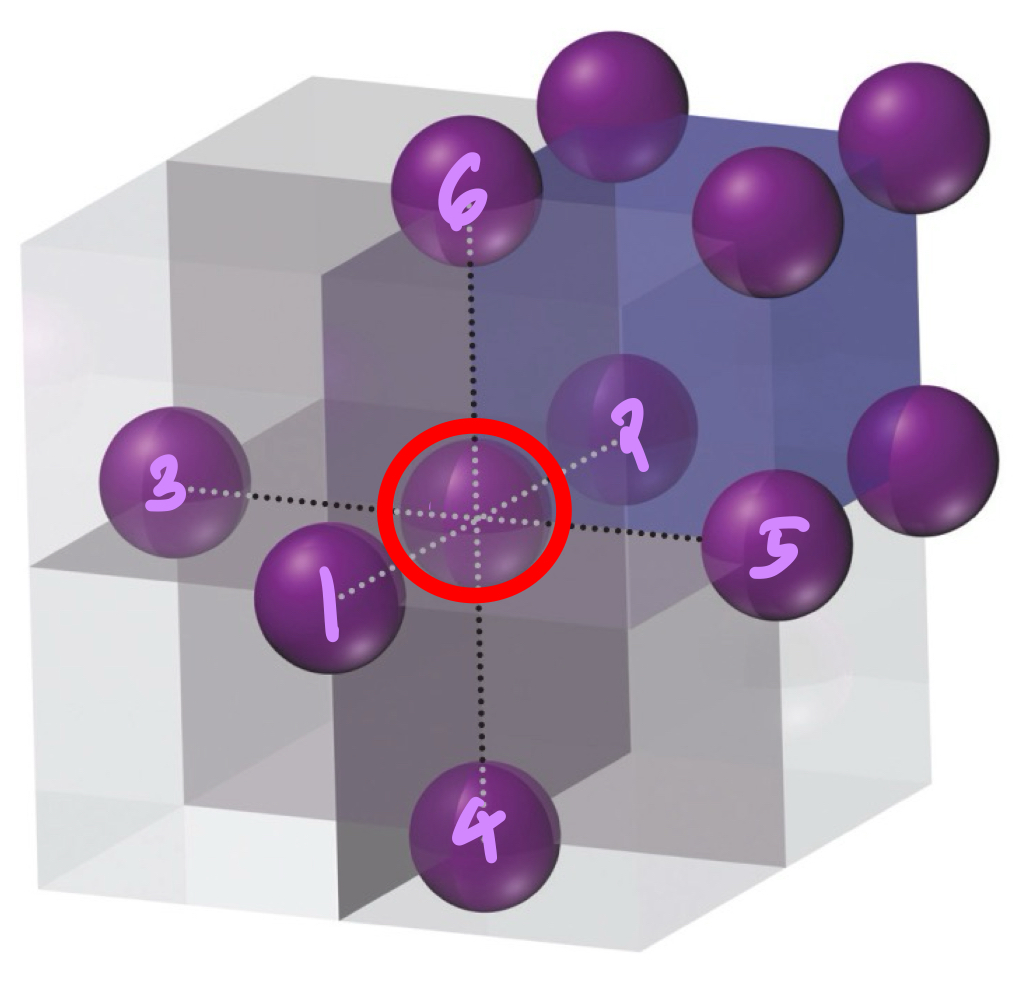
In a primitive unit cell, of a structural element in the centre, what is its coordination number?
6.
What translational symmetry does a P cubic unit cell have?
Translational symmetry with the unit cells next to it.
In a primitive unit cell, of a hole in the centre, what is its coordination number?
8.
Body-centred unit cell
Cubic unit cell with lattice points at the corners and at the centre, then containing a total of 2 lattice points per bcc.
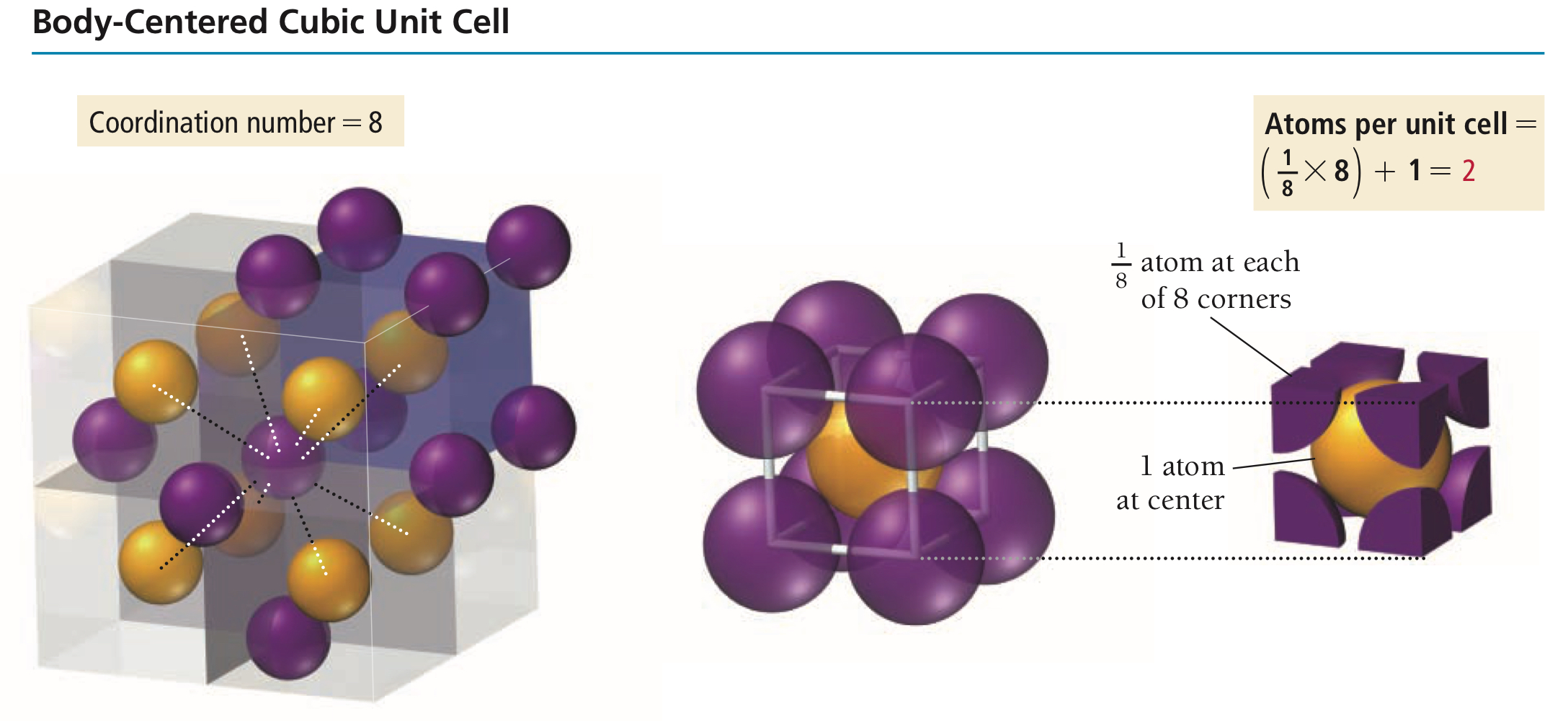
Why does a body-centred unit cell have two lattice points in total?
Take 1/8 from each of the 8 corners, take 1 entire sphere at the centre, and you have a sum of 2.
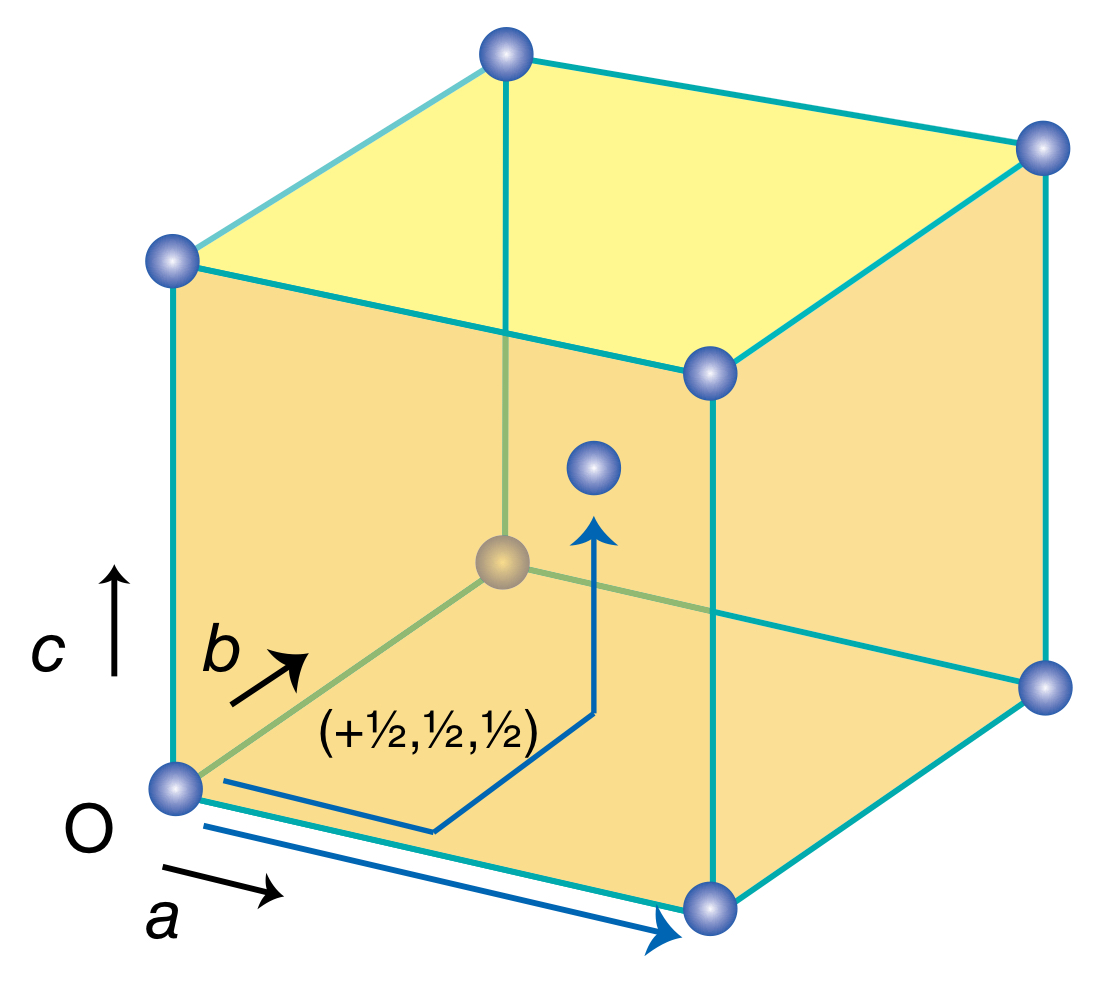
What translational symmetry does a body-centred cubic unit cell have?
It has translational symmetry with the unit cell next to it and from O to +1/2, +1/2, +1/2.
What is the coordination number of each lattice point in a bcc?
8.
Face-centred unit cell
Cubic unit cell with lattice points at the corners and one at the centre of each face.
How many lattice points does a face-centred unit cell have? Explain.
4 lattice points. Take 1/8 of each of the 8 corners, and take 1/2 of each of the 6 faces, you get a sum of 4.
What translational symmetry does a face-centred cubic unit cell have?
Translational symmetry with a unit cell next to it.
Translational symmetry from O to (+1/2, +1/2, 0).
Translational symmetry from O to (+1/2, 0, and +1/2).
Translational symmetry from O to (0, +1/2, +1/2).
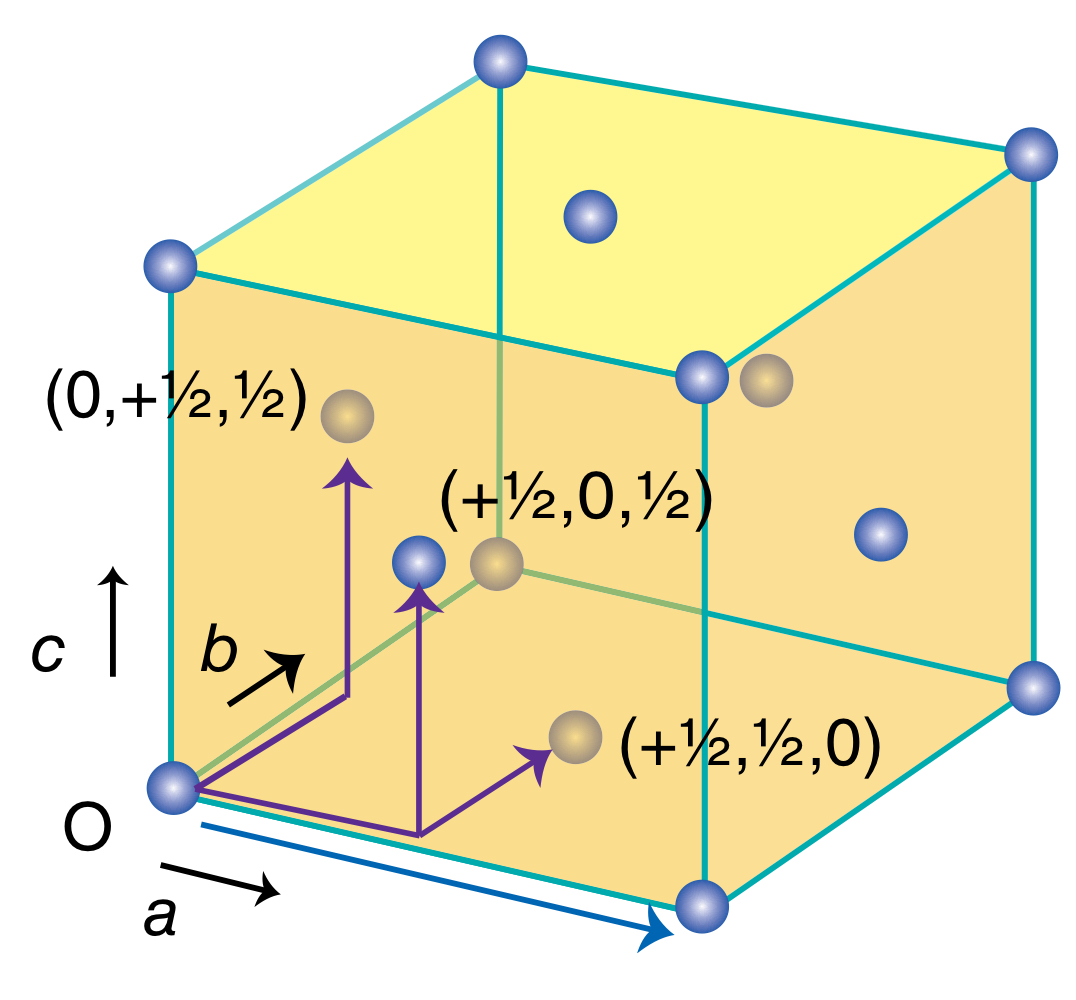
In a face-centred unit cell, how many neighbours does one lattice point have? Rather, what is its coordination number?
12.
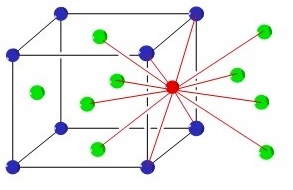
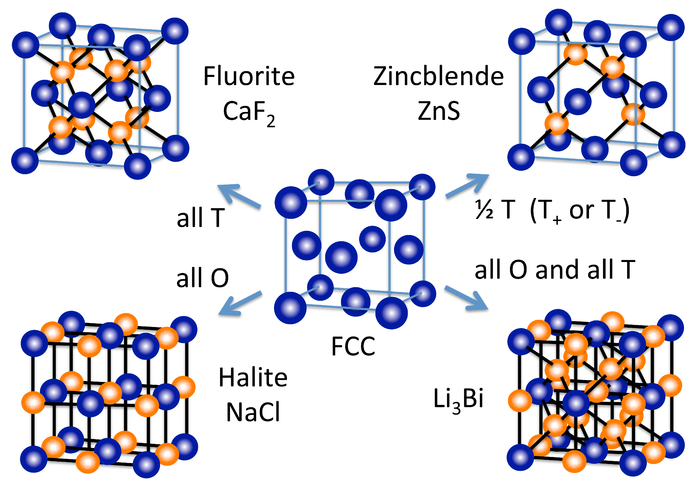
Out of all the three unit cell types, which is the cubic unit cell with the most efficiency in packing, and why?
FCC, because it has the most efficient use of space, using 74% of space and coordinating with 12 atoms.
What are the two types of close-packed systems?
Hexagonal-close packing and cubic close-packing.
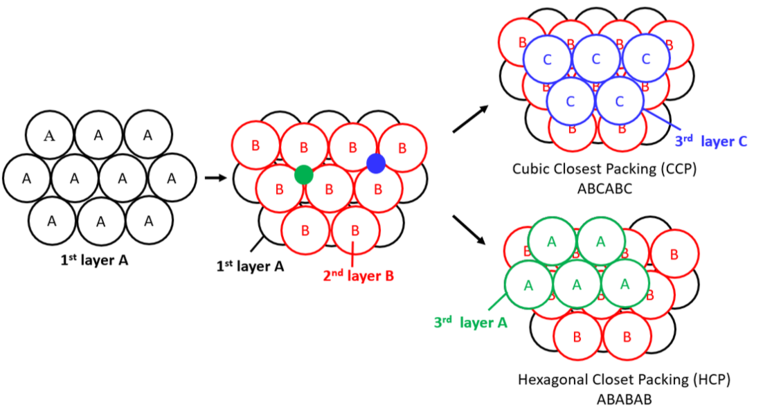
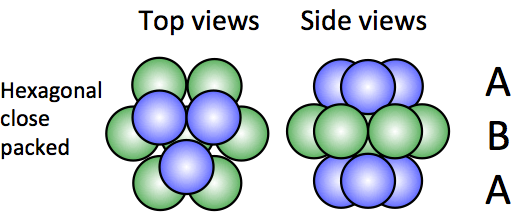
What is hexagonal close-packing, and what is its unit cell?
Close packing wherein you get a layering of A, B, A, B, and so on. Its unit cell is hexagonal.
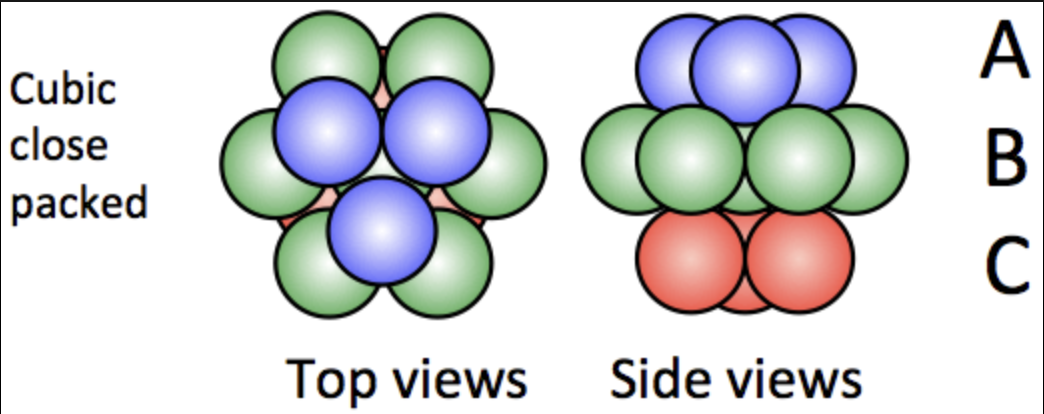
What is cubic close-packing, and what is its unit cell?
Close-packing wherein you get a layering of A, B, C, A, B, C, and so on. Its unit cell is cubic.
Close-packed structures would fill 74% of the space, but 26% is left empty. What do these vacancies represent?
Holes that are filled with a second type of atom, aside from the other type of atom that make up the host lattice.
There are two types of holes in close-packed structures. What are they based on?
Holes are based on the number of lattice points surrounding each hole.
Holes are based on what geometry something might occupy in the hole.
Therefore, what are the two types of holes in close-packed structures?
Octahedral and tetrahedral.
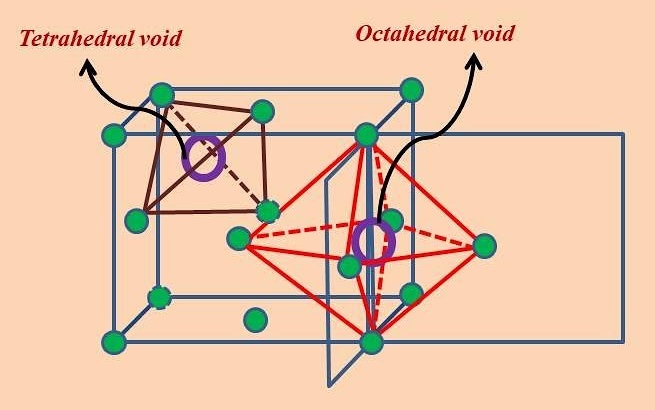
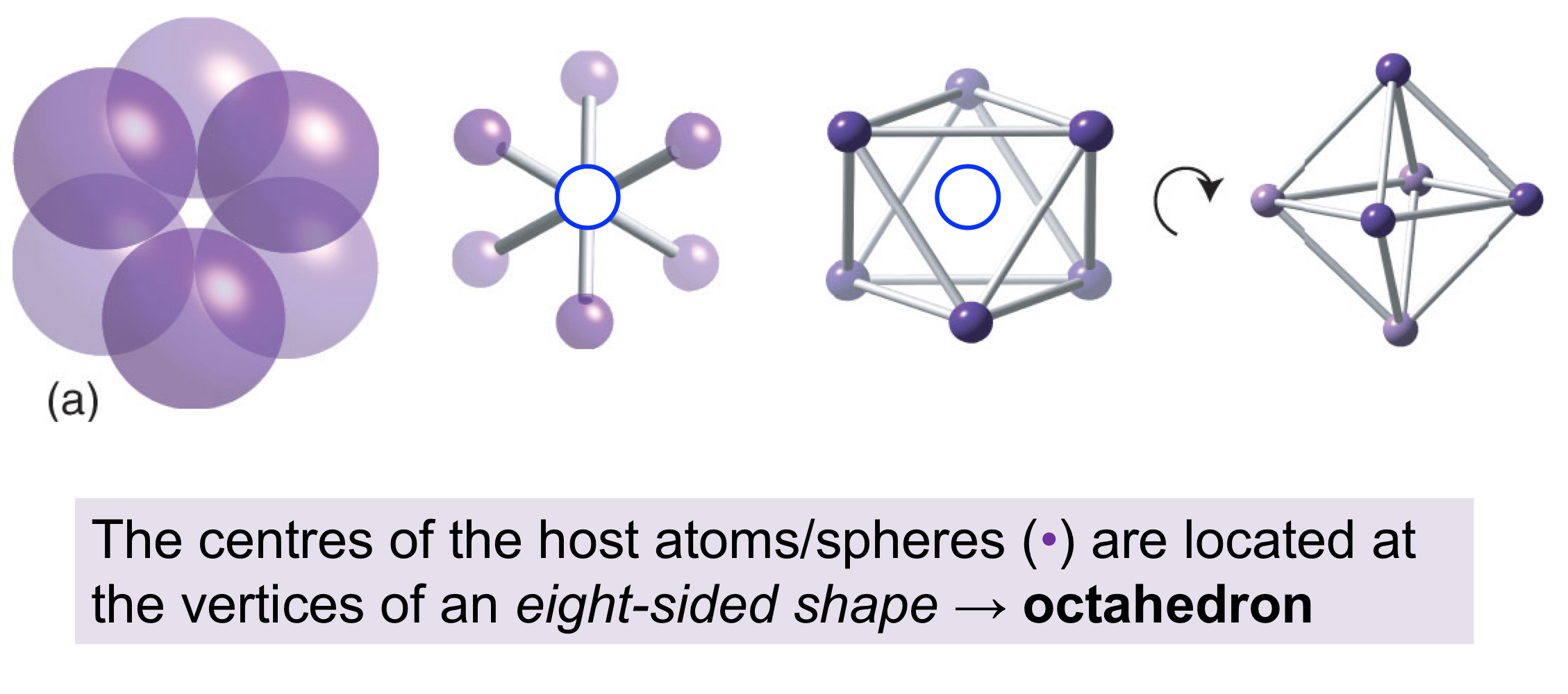
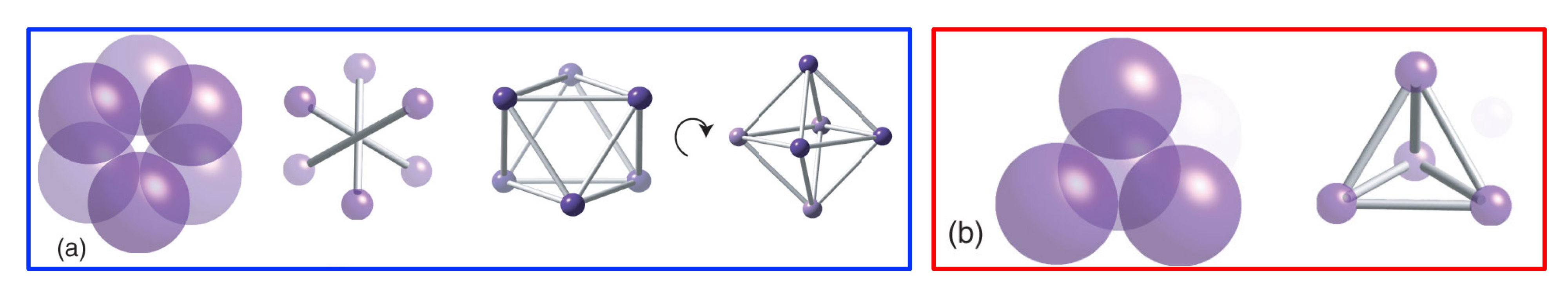
How is an octahedral hole formed?
An arrangement of close-packed spheres with spheres positioned on the top in “non-centre holes,” leaving a hole in the centre, with octahedral geometry and a coordination number of six.
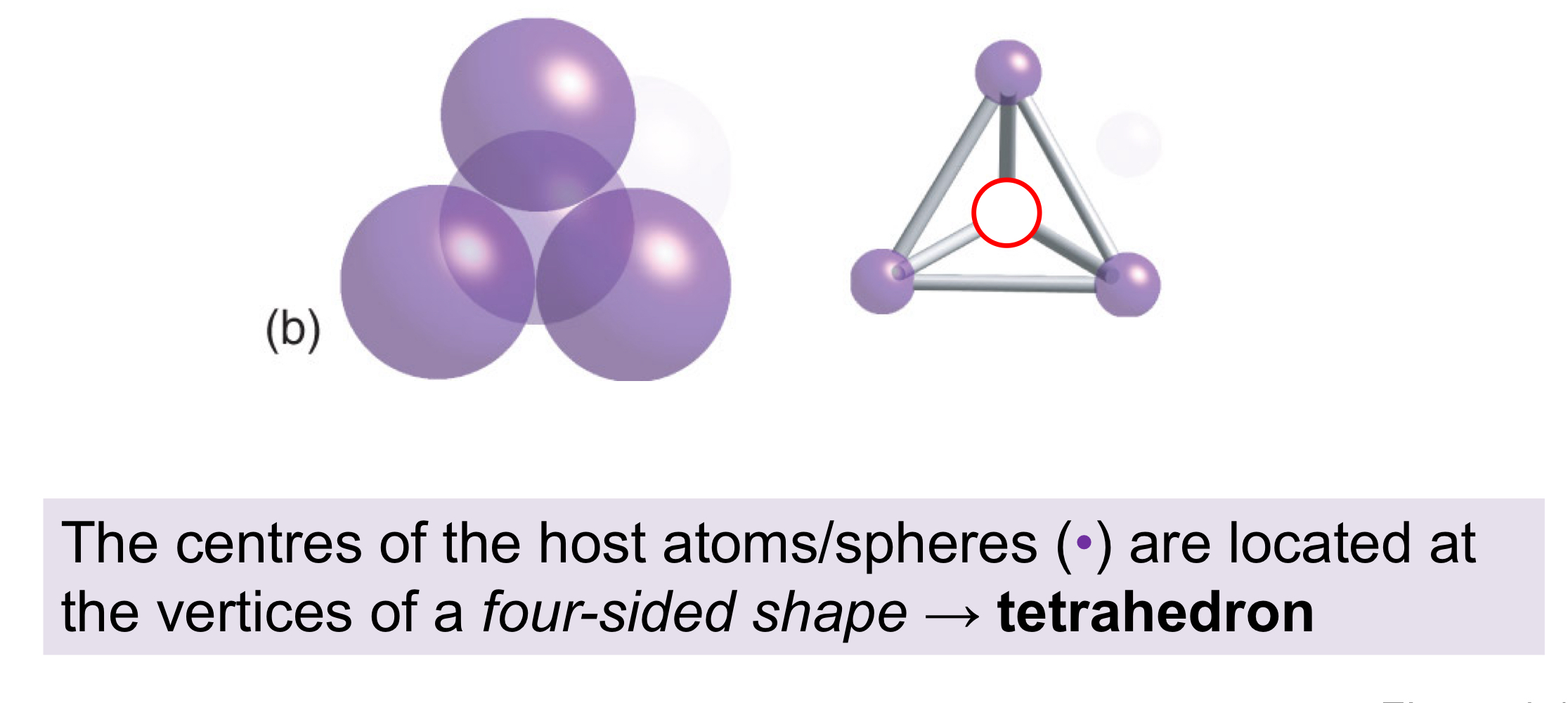
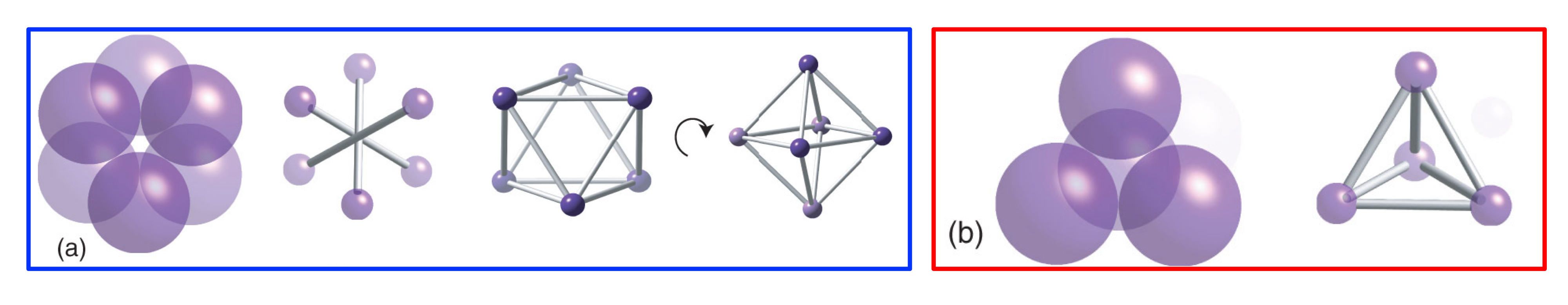
How is an tetrahedral hole formed?
An arrangement of close-packed spheres with a sphere positioned on the top in the "centre” hole, leaving a hole in the centre, with tetrahedral geometry and a coordination number of four.
In a close-packed structure, how many octahedral holes are there, and what is the radius of each octahedral hole?
For every (n) host sphere, there will be (n) octahedral holes, each with a rOh hole of 0.414 x rhost sphere.
In a close-packed structure, how many tetrahedral holes are there, and what is the radius of each octahedral hole?
For every (n) host sphere, there will be (2 x n) tetrahedral holes, each with a rTd hole of 0.225 x rhost sphere.
Where two types of spheres with different radii pack together in a crystalline solid, e.g. cations and anions, what forms the close-packed array, and what occupies the holes?
The larger spheres, usually the anions, form the close-packed array, while the smaller spheres, usually the cations, occupy the holes.
If density is an intrinsic property, then the density of a unit cell is macroscopically equal to the density of what?
The density of the entire material.
What’s the formula for calculating density of a substance from a crystal lattice?
ρ = 4M/(NA)(a3)
To some extent, why is metallic radius useless?
The metallic radius is half the internuclear distance between two metal atoms in a metallic solid. This distance usually increases with increasing coordination number. However, if it adopts a close-packed structure, that would mean that, regardless of the coordination number, it should act as if it had a coordination number of 12.
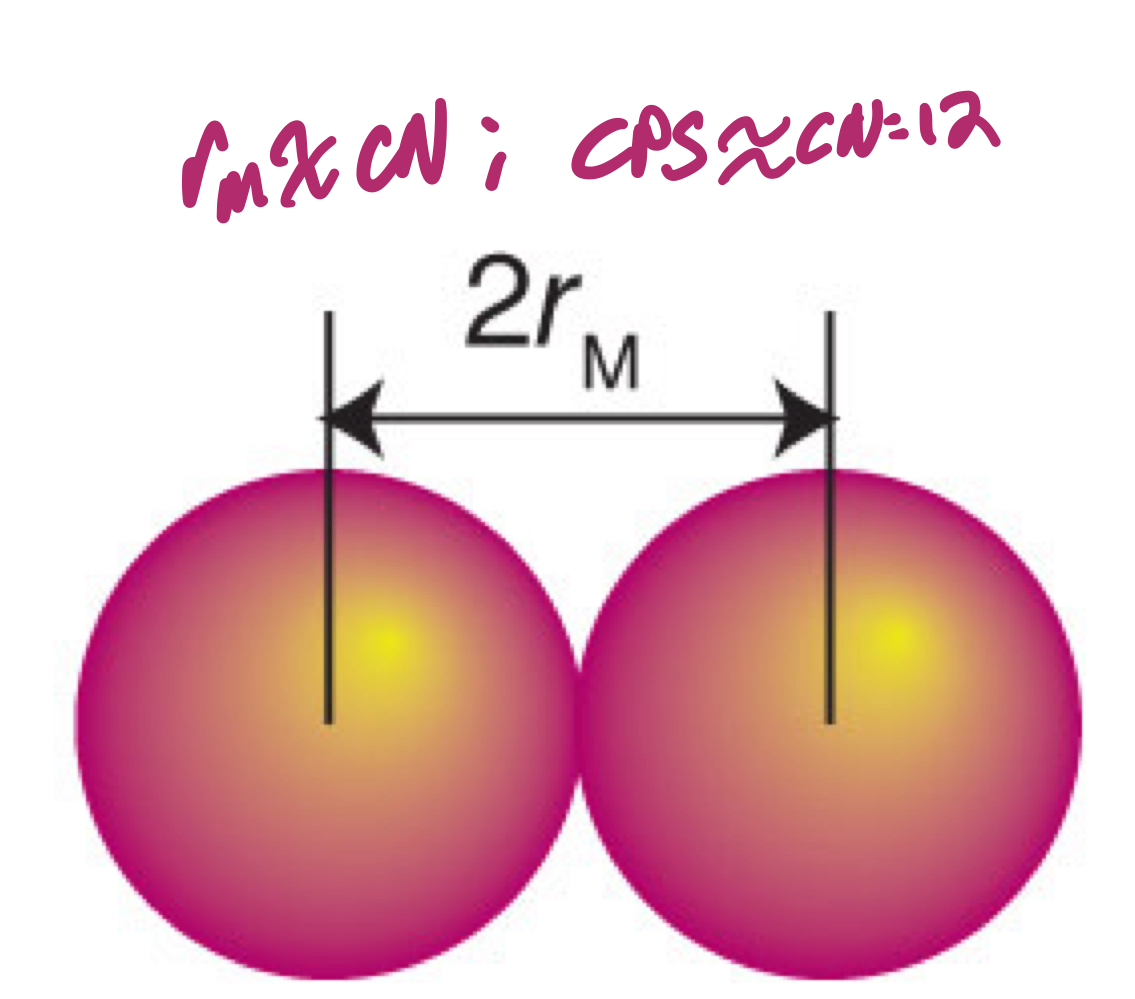
For CN = 12, 8, 6, and 4, what are the relative rm in a close-packed structure, as provided by the Goldschmidt correction?
1, 0.97, 0.96, 0.88, respectively.
How would you calculate the rm using the Goldschmidt correction?
rm = (measured atomic radius)/(Goldschmidt radius with regards to CN of atom)