Cell Bio Lecture 6
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
76 Terms
•The plasma membrane separates chemical reactions of cells
2 types of reactions
•Catabolism – breaks down large molecules
•Anabolism – uses energy to build molecules
Protein Sorting
•Protein synthesis dependent on genetic info:
1.Transcription – DNA copied into mRNA
2.mRNA exits nucleus; moves to ribosome
3.Translation – mRNA used to produce proteins
3 ways to transport proteins into organelles
1.Via nuclear pores
2.Across membranes
3.Via vesicles
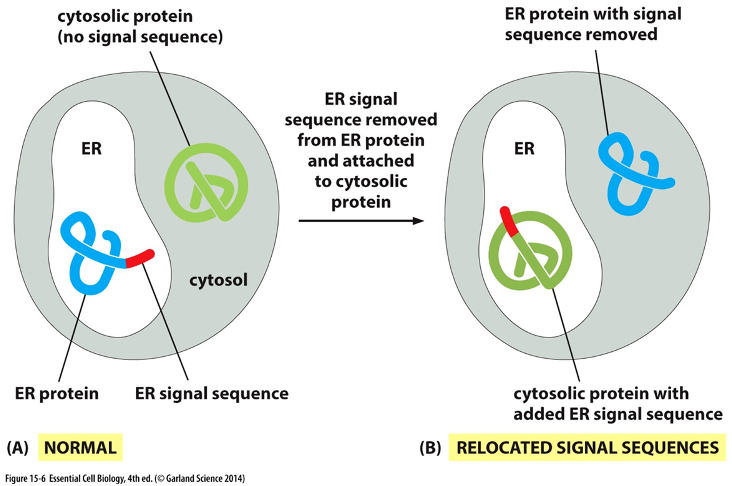
Experiment
•Proteins w/ N-terminal (NH3) signal will go to ER
•Proteins lacking the signal remain in cytosol
•Recombinant DNA technique used to remove signal from ER protein and attach it to cytosolic protein
Result: blue protein trapped in cytosol bc N terminus removed .
Nuclear Envelope
•Double lipid bilayer:
•Inner membrane
•Contains proteins for binding sites of chromosomes
•Outer membrane
•Continuous w/ membrane of the rough ER
•Two membranes fused together at nuclear pores (responsible for importing/exporting to nucelus)
Pores
•Nuclear pores are protein-lined channels in the envelope
•Pores control transport of molecules b/w nucleus & cytoplasm
•Also allow small molecules & ions to pass/diffuse in/out of nucleus
Nuclear import receptors:
•Recognize nuclear localization signal on proteins destined for nucleus
•Direct proteins to pores by interacting w/ fibrils in cytosol
•Penetrate pore by “grabbing” short, repeated amino acid sequences within tangle of pore
GTP Hydrolysis Powers Nuclear Transport
The nuclear import receptor binds to a protein that has a nuclear localization signal.
The receptor-cargo complex moves through the nuclear pore into the nucleus.
Inside the nucleus, Ran-GTP binds to the receptor. This is the key step: the binding of Ran-GTP forces the receptor to release its cargo protein.
The receptor, now carrying Ran-GTP, travels back out into the cytosol.
Once in the cytosol, the GTP is hydrolyzed to GDP. This causes Ran-GDP to dissociate from the receptor, leaving the receptor empty and ready to pick up another nuclear protein for the next round of delivery.
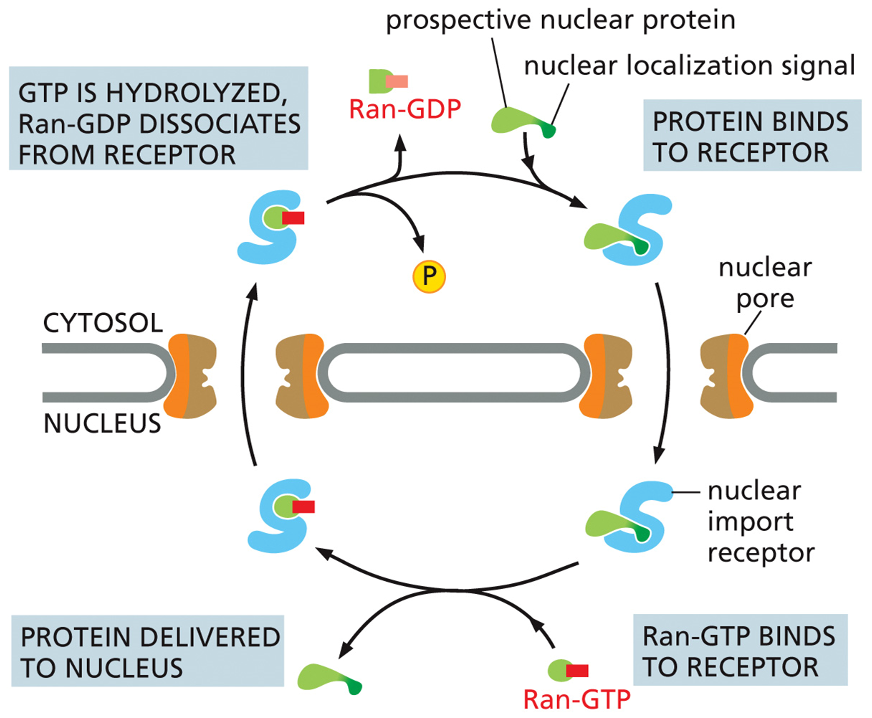
Difference between Ran-GTP and Ran-GDP
Ran-GTP Binding happens: Inside the nucleus.
What it does: Binds to the nuclear import receptor.
The result: Forces the receptor to release its cargo protein into the nucleus.
Its function: Acts as the "drop-off" signal.
Ran-GDP's Role: happens: In the cytosol.
What it does: Dissociates from the import receptor after GTP is hydrolyzed.
result: Frees the receptor so it can pick up new cargo. function: Acts as the "reset" signal.
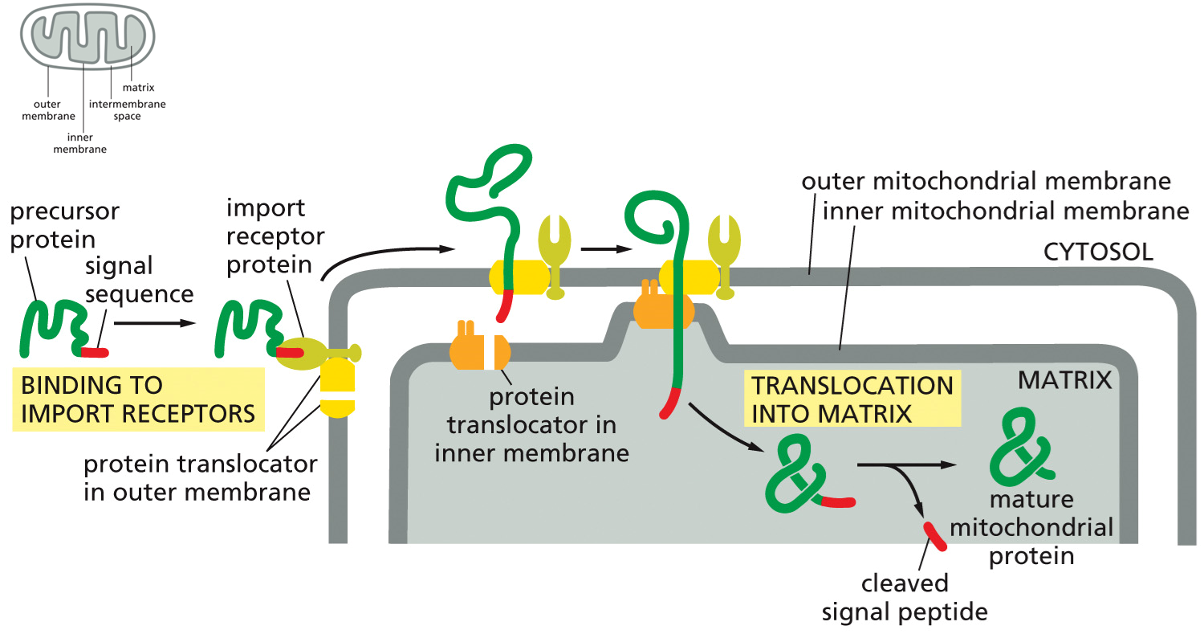
Mitochondrial Protein Import
The precursor protein, which is the protein destined for the mitochondria, is first synthesized in the cytosol. It must remain in an unfolded, linear state to be imported.
Its signal sequence, a specific segment of amino acids, acts as an "address label" that is recognized by the import receptor protein located on the outer mitochondrial membrane.
The import receptor protein's job is to bind to this signal sequence, effectively docking the cargo protein to the surface of the mitochondrion.
The receptor hands the precursor protein over to a protein translocator in the outer membrane. This translocator is a channel that begins guiding the unfolded protein through the first membrane.
Simultaneously, a protein translocator in the inner membrane aligns with the outer one, and together they thread the unfolded protein across both membranes and directly into the mitochondrial matrix
As the protein enters the matrix, a specialized enzyme (a signal peptidase) cleaves off the signal sequence.
Now free of its signal sequence, the polypeptide chain folds into its correct three-dimensional shape, becoming a mature mitochondrial protein ready to perform its function within the organelle.
Peroxisome & Proteins
•Peroxisomes – site of enzymes:
•Catabolism – oxidizes uric acid, amino acids, fatty acids, etc.
•Anabolism – cholesterol, plasmalogens
The Process of Protein Degradation
Tagging for Destruction (Ubiquitin): A target protein is first marked for destruction by having a chain of small proteins called ubiquitin attached to it. This "polyubiquitin chain" acts like a sticky note that says "SHRED THIS".
Recognition and Recycling: The proteasome—the barrel-shaped shredder—has a binding site that recognizes the ubiquitin tag. It grabs the tagged protein, removes the ubiquitin chain to be recycled, and unfolds the target protein.
Degradation: The proteasome then feeds the unfolded protein into its central chamber, which is lined with active proteases (protein-cutting enzymes). These enzymes chop the protein into small fragments, which are then released into the cytosol.
Zellweger Syndrome
•peroxisome dysfunction
•caused by mutations in PEX genes
•progressive degeneration of brain, liver, kidney
•hypotonic, hepatomegaly, seizures, poor feeding
•no cure or treatment
•most do not survive past the first six months of life
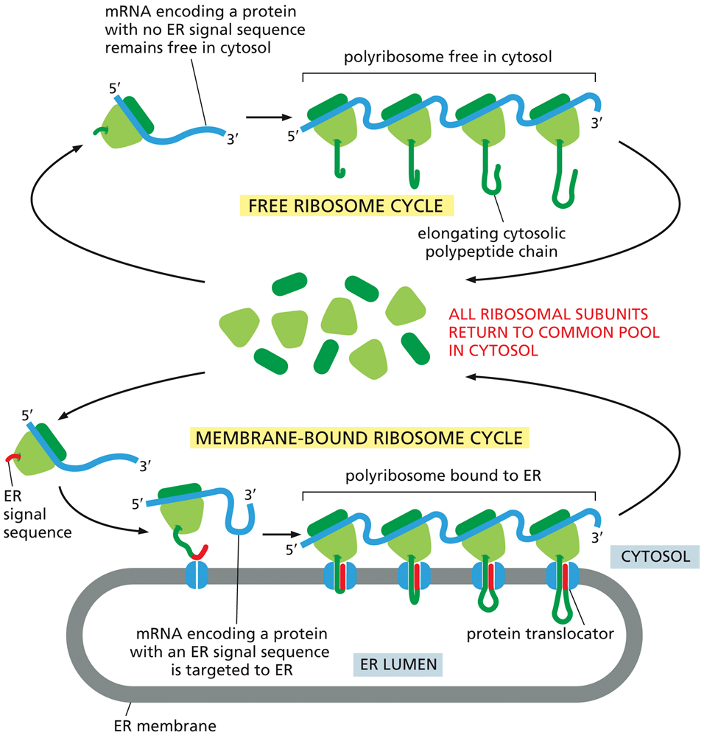
Proteins in Rough ER
•Ribosomes translating proteins with an ER sequence – directed to ER
•Note that many ribosomes bid to each mRNA molecule - polyribosome
Free Ribosome Cycle (Top Pathway)
This pathway is for proteins destined to remain in the cytosol.
An mRNA molecule for a cytosolic protein (one that lacks an ER signal sequence) is grabbed by a ribosome from the common pool.
Translation begins and proceeds entirely in the cytosol.
Multiple ribosomes can translate the same mRNA at once, forming a polyribosome, which efficiently produces many copies of the protein.
The finished proteins are released directly into the cytosol, and the ribosomal subunits detach and return to the common pool.
Membrane-Bound Ribosome Cycle (Bottom Pathway)
This pathway is for proteins that are destined for the endoplasmic reticulum (ER), other organelles, or for secretion from the cell.
Translation begins in the cytosol, just like the free cycle.
However, as soon as the ER signal sequence (the "address label," shown in red) emerges from the ribosome, the entire complex is directed to the ER membrane.
The ribosome docks onto a protein translocator (a channel) in the ER membrane.
As the rest of the protein is synthesized, it is threaded directly through the channel into the ER lumen. This is called co-translational transport.
After translation is complete, the protein is inside the ER, and the ribosome detaches and returns to the common pool in the cytosol.
Vesicular Transport (2 pathways)
•Endocytic pathway (move inside cell)
•ECF molecules ingested in vesicles; delivered to early endosomes, then to lysosomes
Endocytosis: The Import Pathway 📥 "receiving" pathway, used to take in nutrients and other molecules from outside the cell.
Ingestion: The plasma membrane folds inward to form a vesicle, capturing molecules from the extracellular fluid.
Sorting: This vesicle first fuses with an early endosome, which acts as a cellular sorting station.
Degradation: From the endosome, the materials are typically transported to a lysosome. The lysosome contains powerful digestive enzymes that break down the contents for recycling.
•Exocytic pathway
•Proteins transported from ER to Golgi, then to either plasma membrane or lysosomes or released or secreted
Exocytosis: The Secretory Pathway 📦
This is the "shipping out" pathway, used to secrete proteins and deliver new components to the plasma membrane.
Production: The journey begins in the endoplasmic reticulum (ER), where proteins and lipids are synthesized.
Sorting and Packaging: Vesicles carrying these materials bud off the ER and travel to the Golgi apparatus. Here, the molecules are further modified, sorted, and packaged.
Export: Transport vesicles pinch off from the Golgi, travel to the cell surface, and fuse with the plasma membrane, releasing their contents into the extracellular space.
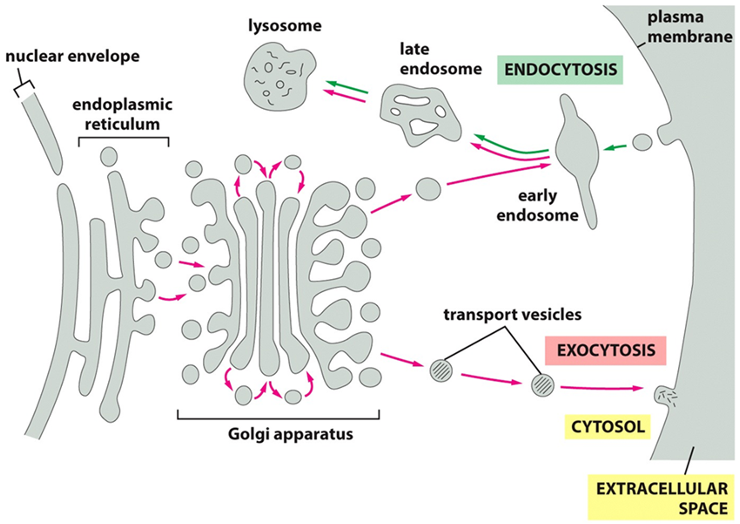
Vesicular Transport
During vesicle transport for endocytosis, 2 coat proteins needed:
•Clathrin – non-specific; comprises the “coat” of vesicle
•Adaptin – secures clathrin to vesicle membrane; helps to select cargo molecules for transport
•Dynamin – GTPase that acts to constrict and “pinch” off vesicle via GTP hydrolysis
•Tethering – Rab proteins on vesicle recognized by tethering protein on target membrane
•Docking – additional recognition via transmembrane proteins v-SNARE (vesicle) & t-SNARE (on target)
•Fusion – v-SNARE & t-SNARE wrap tightly around each other; membrane lipids are continuously fused
The Four Stages of Vesicle Formation
Coat Assembly and Cargo Selection: Cargo molecules bind to their receptors. Adaptin proteins then lock onto the tails of these receptors, beginning the assembly of the clathrin coat on the inside of the membrane.
Bud Formation: As more clathrin molecules are recruited, they self-assemble into a curved, cage-like structure. This physically pulls the plasma membrane inward, forming a "coated pit" that grows into a bud.
Vesicle Formation: The protein dynamin assembles into a ring around the neck of the bud. Using energy, it constricts and pinches the vesicle, releasing it into the cytosol as a complete, clathrin-coated vesicle.
Uncoating: Shortly after its release, the clathrin and adaptin coat is rapidly disassembled. This leaves a "naked transport vesicle" that is now able to fuse with its target destination inside the cell, such as an endosome, to deliver its cargo.
The Step-by-Step Delivery Process
Tethering (The Grab): As the vesicle gets close to its destination, the tethering protein on the target membrane recognizes and snags the specific Rab protein on the vesicle's surface. This initial capture holds the vesicle in place.
Docking (The Lock-in): The tethering protein pulls the vesicle closer to the membrane, allowing the v-SNARE on the vesicle to find and bind tightly to its complementary t-SNARE on the target membrane. This docks the vesicle securely.
Fusion (The Delivery): The v-SNARE and t-SNARE proteins wrap around each other like ropes, a process that provides the force to squeeze out water and merge the two lipid bilayers. This fusion opens a channel, releasing the cargo protein into the target compartment.
Secretion & Glycosylation
•Glycolysation protects proteins from degradation, holds proteins in ER until they’re folded, & serve as transport signal for sorting
•Certain asparagines are glycosylated w/ a branched oligosaccharide
Preparation: A pre-assembled, branched oligosaccharide (a tree made of different sugar molecules like glucose, mannose, and N-acetylglucosamine) is held ready on the ER membrane, anchored by a special lipid called dolichol.
Protein Synthesis: A new protein (the "growing polypeptide chain") is simultaneously being synthesized and threaded through a channel into the ER lumen.
Transfer: An enzyme called oligosaccharyl transferase, which is part of the channel complex, constantly scans the incoming protein. When it recognizes a specific amino acid, asparagine (Asn), it springs into action.
Attachment: The enzyme takes the entire pre-made sugar tree from the dolichol anchor and covalently attaches it to the asparagine on the protein.
This single-step transfer is why it's called N-linked glycosylation—the sugar tree is linked to the nitrogen (N) atom in asparagine's side chain.
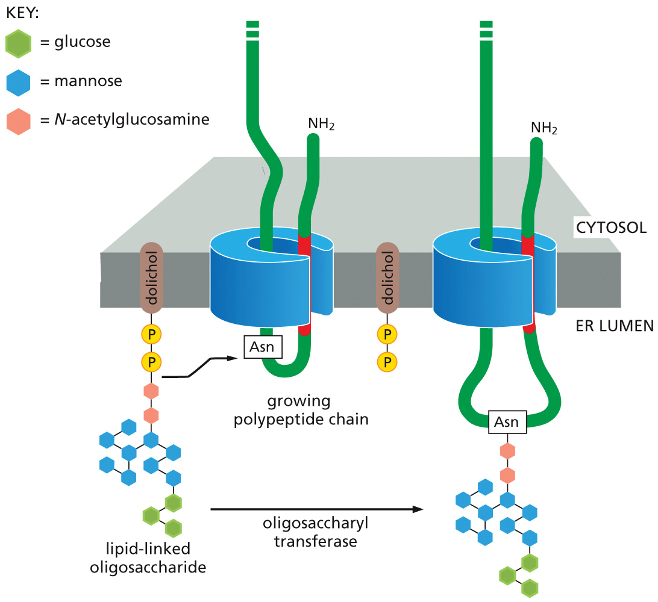
Secretion
•Only properly-folded proteins can exit ER
•Chaperones – aid proteins in folding
Proteins that fail to fold & assemble correctly are degraded
Cystic Fibrosis
•Mutations in CFTR (cystic fibrosis transmembrane conductance regulator) gene
•CFTR normally in membranes of cells to produce mucus, sweat, saliva, digestive enzymes
•Mutated? Impaired transport of Cl- & H2O in and out of cells; cells in lungs produce mucus that is abnormally thick
Individuals w/ cystic fibrosis have very salty sweat due to lack of reabsorption of Cl- back into cells, leading to higher Cl- in sweat and salty sweat
Impaired movement of water to outside of lung cells – sticky, thick mucus and lack of clearance of bacteria from respiratory tract
This image breaks down the normal function of the CFTR gene, from its genetic origin to its role as a crucial channel in our cells.
From Gene to Function
The diagram illustrates a three-step process:
Inheritance: A child inherits two copies of Chromosome 7, one from each parent. This chromosome contains the genetic blueprint for many proteins, including CFTR.
Protein Production: A specific section of Chromosome 7, the CFTR gene, holds the instructions to create the CFTR protein. This protein folds into a channel that is placed on the cell's surface.
Chloride Transport: The normal function of this channel is to transport chloride ions (and by extension, water) in and out of the cell. This is vital for maintaining the right balance of salt and water in secretions like mucus, sweat, and digestive enzymes.
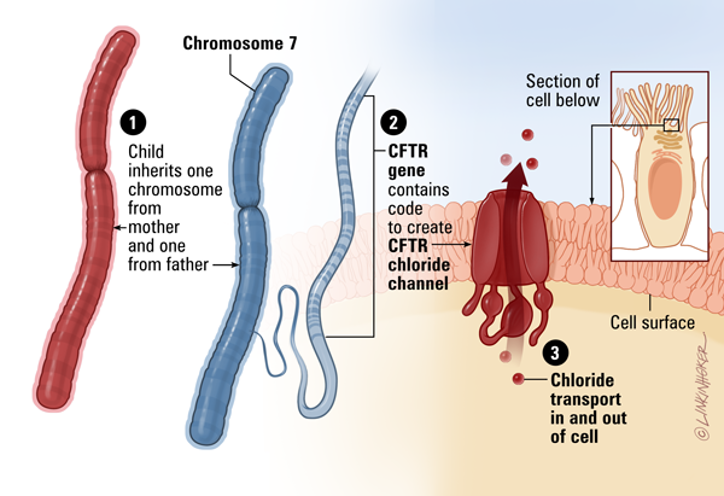
Secretion
•Unfolded Protein Response (UPR)
1)Accumulation of misfolded proteins
2)Different transmembrane sensor proteins serve different UPR activities
•Some proteins stimulate transcription regulators
•Some acts to inhibit protein synthesis
3) Activation of cell death mechanism (apoptosis)
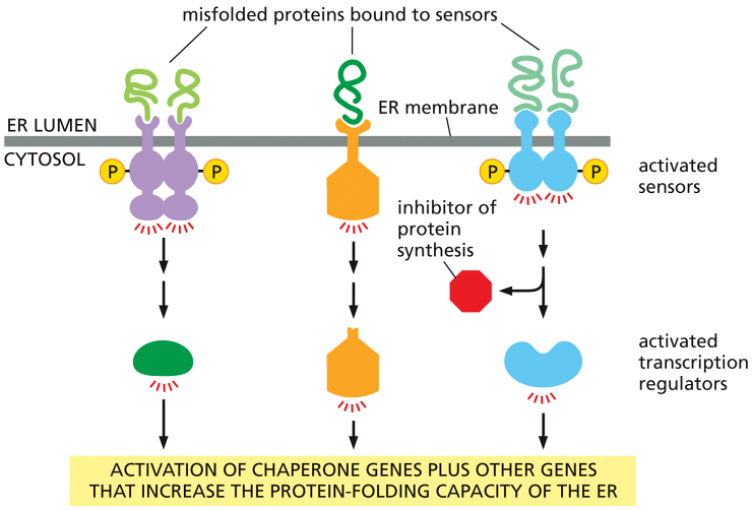
The Cell's Response to ER Stress
Think of the ER as a factory assembly line for folding proteins. When unfolded proteins start to pile up, the factory manager (the UPR) initiates a multi-step plan to resolve the issue.
Sounding the Alarm: Special sensor proteins embedded in the ER membrane detect the accumulation of misfolded proteins in the ER lumen and become activated.
Taking Action: As shown in the diagram, these activated sensors trigger several parallel responses simultaneously:
Increase Folding Capacity: They activate transcription regulators. These molecules travel to the nucleus to switch on genes that produce more chaperone proteins and other helpers, essentially calling in more "workers" to help fold the backlog of proteins.
Reduce the Load: At the same time, another pathway is activated to temporarily inhibit protein synthesis. This slows down the "conveyor belt," reducing the flow of new proteins into the already stressed ER.
The overall goal of the UPR is to restore balance by matching the ER's protein-folding capacity to the workload.
Golgi apparatus composed of
•cisternae
•2 faces to Golgi:
•Cis – entry face adjacent to ER
Trans – exit face & points toward plasma membrane
Exocytosis Types 2 types
•Constitutive (unregulated)
•Soluble proteins continually secreted from cell
•Regulated
•Secretory vesicles fuse with plasma membrane to release proteins when cell receives ECF signal (has to be tightly controlled)
Blood Glucose & Insulin
•Exocytosis-regulated secretion
•Increased blood glucose stimulates pancreatic beta cells to secrete insulin
Types of Endocytosis
•Phagocytosis – cell eating & ingestion of large particles (cell debris, microorganisms)
•Pinocytosis – cell drinking & ingestion of fluid and molecules (observed in small intestine)
•Receptor-mediated – clathrin-mediated
Phagocytosis
•Phagocytes:
•Macrophages
•Neutrophils
•Monocytes
•Dendritic cells
•Functions:
•defend against infection by ingesting invading microorganisms
•scavenge dead and damaged cells and cell debris
The Steps of Phagocytosis
Chemotaxis and Adherence (Find and Stick): The phagocyte is first chemically attracted to the microbes (chemotaxis). Once it reaches them, its plasma membrane attaches to the microbe's surface (adherence).
Ingestion (Engulf): The phagocyte extends its membrane projections, called pseudopods, to surround and engulf the microbes. This brings the microbes inside the cell, enclosed within a membranous sac called a phagosome.
Formation of Phagolysosome (Prepare to Digest): The phagosome moves deeper into the cell and fuses with a lysosome, an organelle filled with powerful digestive enzymes. The resulting combined vesicle is called a phagolysosome.
Digestion (Kill and Dismantle) 💥: Inside the phagolysosome, the digestive enzymes and other chemicals break down and destroy the ingested microbes.
Elimination (Dispose) 🗑: Any indigestible material left over is contained within a residual body, which then moves to the cell surface and expels its contents to the outside through exocytosis.
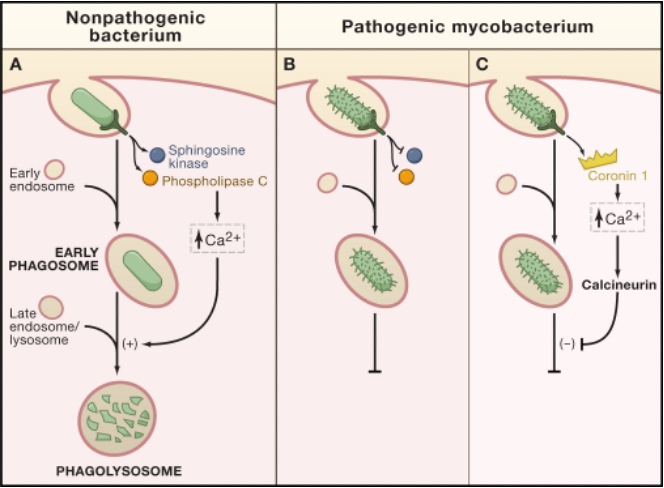
Phagocytosis – Clinical Correlate
•Mycobacterium tuberculosis
•bacterial components prevent fusion with late endosomes/lysosomes
•the engulfed organism survives and multiplies within the macrophage
A: The Normal Response (Nonpathogenic Bacterium)
When a phagocyte engulfs a harmless bacterium, the process works as intended:
A phagosome is formed around the bacterium.
This triggers a signaling cascade that increases intracellular calcium (Ca2+).
The calcium signal promotes the fusion of the phagosome with a lysosome.
The resulting phagolysosome uses its digestive enzymes to destroy the bacterium.
Panels B & C: The Evasion Strategy (Pathogenic Mycobacterium)
Pathogenic mycobacteria have evolved to disrupt this process and survive inside the very cells that are meant to kill them.
After being engulfed, the mycobacterium actively blocks the signaling pathway.
As shown in Panel C, it can use proteins like Coronin 1 to specifically inhibit the calcium signal required for fusion.
By preventing the phagosome from fusing with the lysosome, the bacterium avoids destruction. It creates a protected niche inside the phagocyte, where it can survive and multiply.
Pinocytosis
•Moves liquid & solutes into cells
•Example – engulfing of fat droplets in small intestine
Receptor-Mediated Endocytosis
•Selective uptake of extracellular molecules via binding to specific cell surface receptors
•E.g. – ingestion of cholesterol by mammalian cells
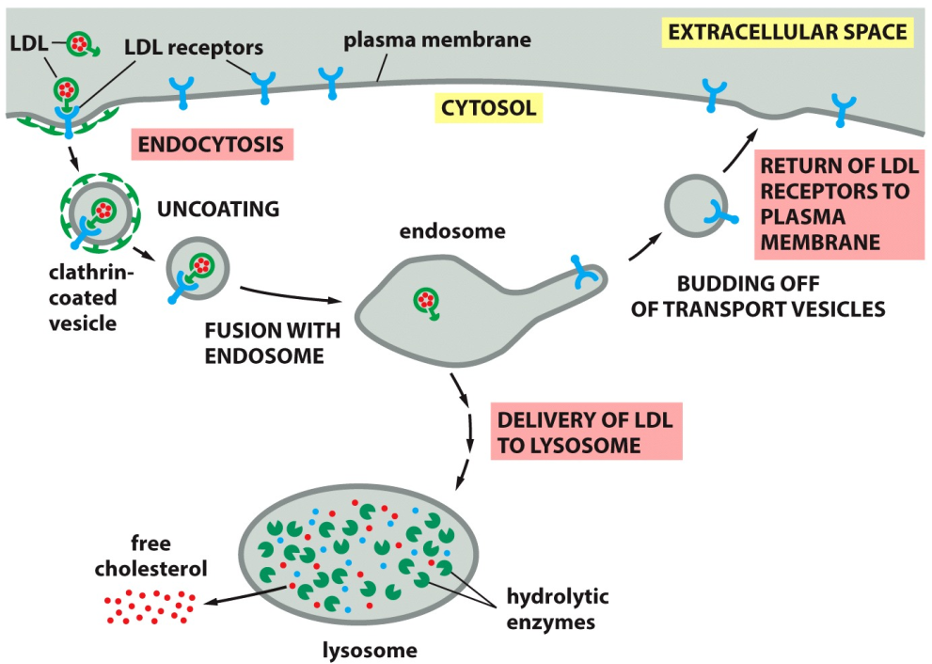
Cholesterol Endocytosis
The Pathway for Cholesterol Uptake
Binding and Capture: LDL particles, which are rich in cholesterol, circulate in the bloodstream. They bind to specific LDL receptors on the cell's surface. This binding triggers the formation of a
clathrin-coated vesicle that brings the LDL and its receptor into the cell.
Sorting: The vesicle sheds its clathrin coat and fuses with an endosome, a mildly acidic sorting organelle within the cell. The acidic environment causes the LDL particle to detach from its receptor.
Receptor Recycling: This is a key step for efficiency. The now-empty LDL receptors are sorted into new transport vesicles that bud off from the endosome and return to the plasma membrane, ready to capture more LDL.
Cholesterol Release: The LDL particle is transported from the endosome to the lysosome. Here, powerful hydrolytic enzymes break down the LDL, releasing
free cholesterol into the cytosol where the cell can use it.
Familial Hypercholesterolemia
•LDL receptor mutations (>1000)
•receptors are missing or nonfunctional
•cholesterol accumulates in the blood stream and predisposes the person to atherosclerosis and heart attack
•blood cholesterol level 1000 mg/dL(
•normal < 200mg/dL )
ormal Pathway
On the left, the diagram shows the healthy, functional process:
LDL receptors are produced inside the cell and placed on the cell surface.
These receptors recognize and bind to LDL cholesterol (LDL-C) particles circulating in the blood.
The receptor-LDL complex is then taken into the cell (endocytosis), effectively clearing cholesterol from the bloodstream.
Defective Pathway (LDLR-)
The panel on the right illustrates what happens when there is a mutation in the LDL receptor gene (LDLR-).
The Defect: The cell produces LDL receptors that are structurally or functionally defective. As the text states, there is
"No binding of LDL particles to [the] defective LDL receptor".
The Consequence: Because the liver cells cannot grab onto and remove LDL from the blood, the cholesterol is left to accumulate in the circulation. This leads to dangerously high blood cholesterol levels and a significantly increased risk of atherosclerosis and heart attacks.
![<p><span>•LDL receptor mutations (>1000)</span></p><p><span>•receptors are missing or nonfunctional</span></p><p><span>•cholesterol accumulates in the blood stream and predisposes the person to atherosclerosis and heart attack</span></p><p><span>•blood cholesterol level 1000 mg/dL(</span></p><p><span>•normal < 200mg/dL )</span></p><p>ormal Pathway</p><p></p><p>On the left, the diagram shows the healthy, functional process:</p><ol><li><p>LDL receptors are produced inside the cell and placed on the cell surface.</p></li><li><p>These receptors recognize and bind to LDL cholesterol (LDL-C) particles circulating in the blood.</p></li><li><p>The receptor-LDL complex is then taken into the cell (endocytosis), effectively clearing cholesterol from the bloodstream.</p></li></ol><p></p><p>Defective Pathway (LDLR-)</p><p></p><p>The panel on the right illustrates what happens when there is a mutation in the LDL receptor gene (LDLR-).</p><ul><li><p><strong>The Defect:</strong> The cell produces LDL receptors that are structurally or functionally defective. As the text states, there is</p><p><strong>"No binding of LDL particles to [the] defective LDL receptor"</strong>.</p><p></p></li><li><p><strong>The Consequence:</strong> Because the liver cells cannot grab onto and remove LDL from the blood, the cholesterol is left to <strong>accumulate in the circulation</strong>. This leads to dangerously high blood cholesterol levels and a significantly increased risk of atherosclerosis and heart attacks.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/17d84935-7fd6-4a20-8261-b3ee68c8ef13.png)
Endosomes
•Routes taken by receptor once they have entered an endosome:
1.Recycle: most often occurs
2.Degrade in lysosome
3.Transcytosis (receptor goes to another site of plasma membrane)
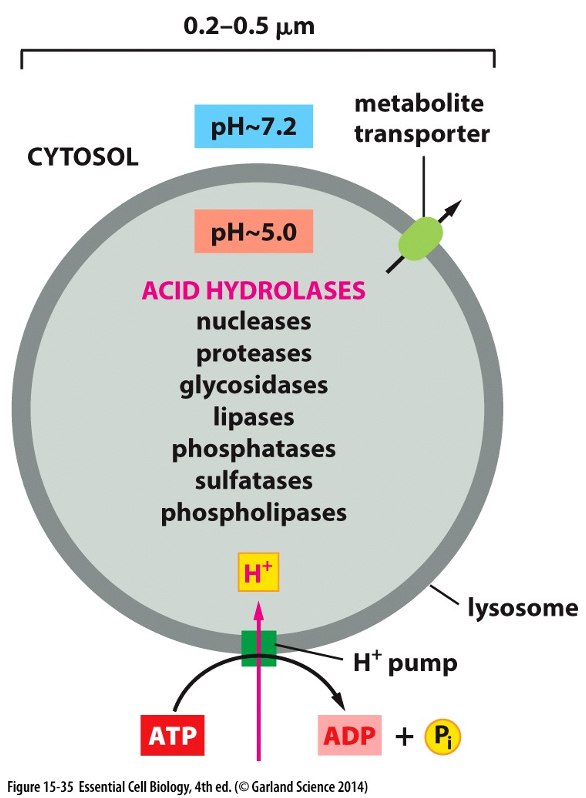
Lysosomes
•Lysosomes contain about 40 types of hydrolytic enzymes
•Enzymes are optimally active in the acidic condition (PH - ~5) maintained within lysosomes by ATP-driven H+ pump
•Transporters on the lysosomal membrane allow the final products to be transferred to the cytosol; from there, they can be either excreted or utilized by the cell.
Lysosome-Autophagy
•Autophagy: mechanism by which a cell will “eat itself”
•Digesting molecules and organelles that are damaged or obsolete
•Cell’s recycling system to recycle damaged cell parts into functioning parts
Signaling Principles
•Cells communicate via signaling molecules
•Sending cell produces chemical signals
•Ligands – proteins, peptides, amino acids, gas, nucleotides, steroids
•Target cells have appropriate receptor to bind to signal
•Receptors – bind to ligands in specific manner & mediate biochemical and physiological changes
Endocrine Signals
•Endocrine cells – produce chemical hormones
•Hormones transported in the blood & stimulate specific cells (insulin binds to liver cells, skeletal muscle cells
Paracrine Signals
•Signals released by cells to stimulate nearby cells
•E.g. – wound healing
•Epidermal growth factor stimulates epidermal cells to proliferate
Autocrine Signals
•Signals released by cells to stimulate that same cell
•Observed in T cell lymphocytes to allow for self-stimulation
Synaptic Signals
•At synapse – electrical signal traveling to end of presynaptic neuron converted to chemical signal to stimulate postsynaptic neuron
Contact-Dependent
•Cells make direct contact
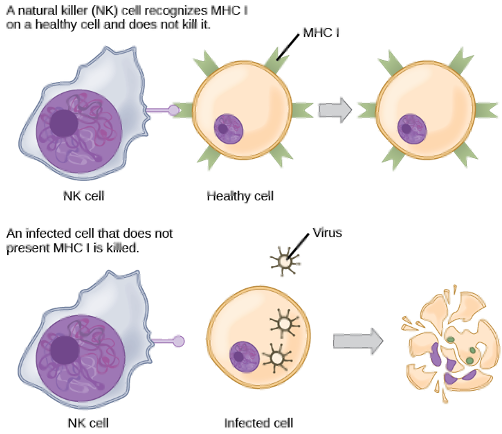
•E.g. – NK cells use MHC to recognize “self” cells and cells infected by pathogens
Top: Recognition of a Healthy Cell ✅
In this scenario, the NK cell on the left interacts with a healthy body cell.
The healthy cell displays specific "self" markers on its surface (like MHC proteins).
The NK cell recognizes these markers, which sends an inhibitory signal telling it "this cell is friendly." The NK cell disengages, and the healthy cell is left unharmed.
Bottom: Killing of an Infected Cell ❌
In this scenario, a cell has been infected by a pathogen (like a virus).
Many infected or cancerous cells stop displaying the normal "self" markers on their surface.
The NK cell recognizes the absence of these inhibitory signals. This lack of a "don't kill me" signal activates the NK cell, which then releases cytotoxic molecules that kill the infected cell, causing it to break apart.
Notch Signaling
•Membrane-bound Delta signal protein binds to Notch (Delta receptor)
•Notch is cleaved; part of receptor transfers to nucleus to act as a transcriptional regulator
Binding: The process begins when a Delta signal protein on the surface of one cell (the signaling cell) binds to a Notch receptor on an adjacent cell (the receiving cell/ target)
Cleavage: This binding event triggers the Notch receptor to be cleaved, or cut. A piece of the receptor's intracellular domain, called the "cleaved Notch tail," is released into the cytosol.
Activation: This cleaved Notch tail then travels directly into the nucleus, where it acts as a transcription regulator, binding to DNA and activating the transcription of specific target genes.
Extracellular Signals Bind to Receptors
Cell-Surface Receptors
Location: These receptors are embedded in the cell's plasma membrane, with the binding site facing the outside.
Signal Type: They are used for signal molecules that are generally large or hydrophilic (water-soluble) and cannot pass through the cell membrane on their own.
Mechanism: The external signal molecule binds to the receptor, causing a change on the inside of the cell. This change generates new intracellular signaling molecules, which then relay the message throughout the cell. The original signal never has to enter.
Intracellular Receptors
Location: These receptors are located inside the cell, either in the cytosol or the nucleus.
Signal Type: They are used for small, hydrophobic (lipid-soluble) signal molecules, such as steroid hormones, which can diffuse directly across the plasma membrane.
Mechanism: The signal molecule enters the cell and binds directly to its receptor. This activated receptor-signal complex can then carry out its function, such as moving into the nucleus to directly regulate gene transcription.
Features of Cell Signaling
(A) In a Heart Pacemaker Cell: When acetylcholine binds to its receptor, it causes a decreased rate of firing. This acts to slow down the heart rate.
(B) In a Salivary Gland Cell: The same signal molecule binding to a receptor on this cell triggers secretion, causing the release of saliva.
(C) In a Skeletal Muscle Cell: Acetylcholine binds to a different type of receptor, which acts as an ion channel. This binding causes the muscle cell to contract.
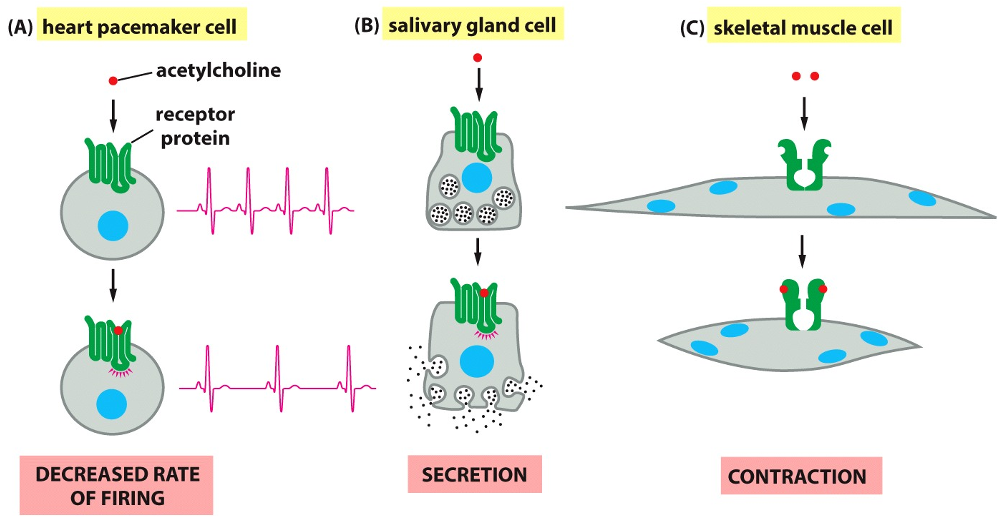
Signal Effects on Cells
•Every cell type responds to a specific set of signals
•Cells express a set of receptors to elicit a response
•Signals work in combinations to regulate cell behaviors
Early vs. Late Cell Responses
•Fast – no synthesis of new protein
•Involved in cell movement, secretion, metabolism
•Slow – involves changes in gene expression and protein synthesis
•Involved in cell differentiation, growth, division
Intracellular Signaling
•Effector: downstream molecules that generate a response
•Second messenger:
•Diffuse through a cell and convey information to a wide variety of targets
•cAMP, cGMP, Ca2+, inositol triphosphate (IP3), diacylglycerol (DAG), NO
The Three Stages of Cell Signaling
Reception: An extracellular signal molecule acts as the initial message. It binds to a specific receptor protein on the cell's surface. This is the first step in receiving the signal.
Transduction: The binding event activates the receptor, which in turn activates the first in a series of intracellular signaling molecules. These molecules then activate the next in a chain reaction, or cascade. This process relays the signal from the outside to the inside of the cell, often amplifying it along the way.
Response: The last molecule in the cascade activates effector proteins, which are the molecules that actually carry out the cell's response. As shown in the diagram, a single signal can be distributed to different effectors to produce multiple outcomes simultaneously:
Altered Metabolism: By activating a metabolic enzyme.
Altered Cell Shape or Movement: By acting on a cytoskeletal protein.
Altered Gene Expression: By activating a transcription regulator, which controls which genes are turned on or off.
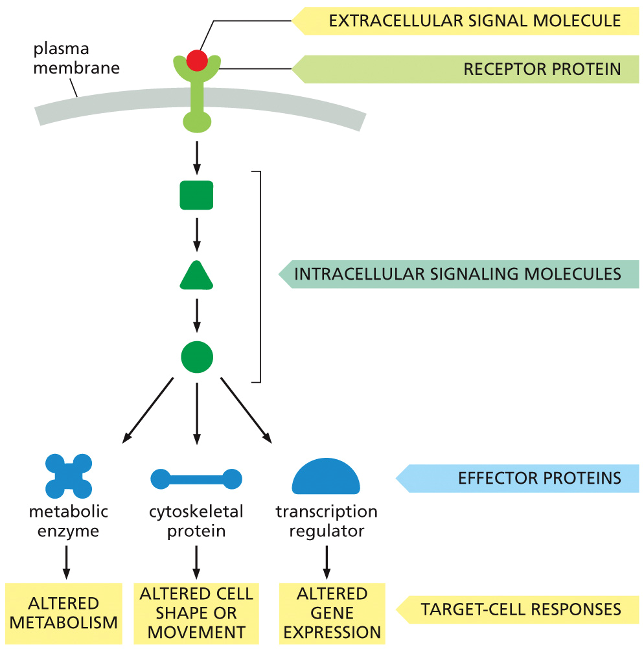
Intracellular Signaling Pathways-crucial functions
One or more crucial functions:
1.relay signal onward and help spread signal through the cell
2.amplify signal received
3.integrate intracellular signaling pathways
4.distribute signal to more than one effector protein
5.modulate the response to the signal by feedback
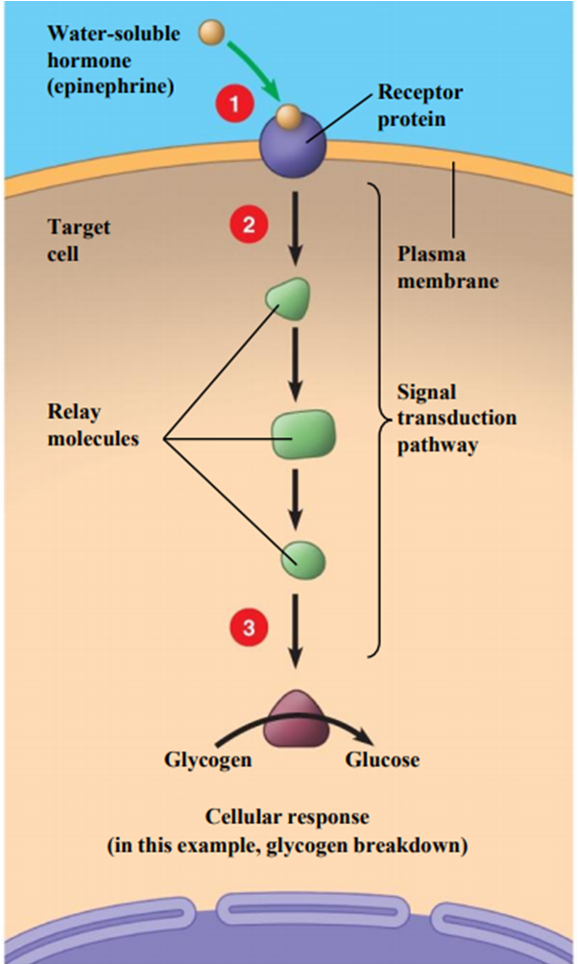
signal transduction pathway for the hormone epinephrine (adrenaline), illustrating how it triggers the breakdown of glycogen into glucose in a target cell.
Reception: The water-soluble hormone, epinephrine, cannot pass through the cell's plasma membrane. Instead, it binds to a specific receptor protein on the cell's outer surface.
Transduction: This binding event activates the receptor, which in turn triggers a signal transduction pathway inside the cell. This pathway consists of a series of relay molecules that pass the message from one to the next, often amplifying the signal at each step.
Response: The last relay molecule in the chain activates an enzyme that carries out the final cellular response. In this example, the enzyme breaks down stored glycogen into glucose, which can then be used by the body for a quick burst of energy.
Feedback Mechanisms
•Two types of feedback regulations
•positive feedback
•example: lactation
•baby suckles on the nipple
•nerve response produces more prolactin
•negative feedback
•example: baroreflex regulation of blood pressure
•high blood pressure decreases sympathetic nerve activity, slows down heart rate and contractility
Molecular Switches
•Phosphorylation
•kinase activate
•serine/threonine kinase
•tyrosine kinase
•phosphatase inactivate
Turning the Switch ON (Phosphorylation)
An incoming signal ("SIGNAL IN") activates an enzyme called a protein kinase.
The protein kinase takes a phosphate group from an ATP molecule and attaches it to the target protein.
This addition of a phosphate group (phosphorylation) changes the protein's shape and activity, flipping it to the "ON" state.
Once "on," the protein can now relay the signal to other components in the cell ("SIGNAL OUT").
Turning the Switch OFF (Dephosphorylation)
To terminate the signal, a different enzyme called a protein phosphatase removes the phosphate group from the protein.
This removal (dephosphorylation) causes the protein to revert to its original, inactive "OFF" state, stopping the signal.
GTP-binding proteins
Turning the Switch ON (GTP Binding)
The cycle begins with the G-protein in its "OFF" state, bound to a molecule of GDP.
An incoming signal ("SIGNAL IN") causes the G-protein to release its GDP and bind to a different molecule, GTP.
This exchange of GDP for GTP activates the protein, flipping it to the "ON" state.
In its "on" state, the protein can now relay the signal to other components in the cell ("SIGNAL OUT").
Turning the Switch OFF (GTP Hydrolysis)
The G-protein has a built-in timer. It has an intrinsic enzymatic activity that allows it to hydrolyze its own GTP.
As shown in the chemical diagram on the left, this hydrolysis reaction removes a phosphate group from GTP, converting it back to GDP.
Once the protein is bound to GDP again, it reverts to its inactive "OFF" state, automatically terminating the signal.
Steroid Hormones as Signals
•Small hydrophobic hormones (steroids) diffuse through plasma membrane and bind to intracellular receptors
•Receptors act as transcription regulators
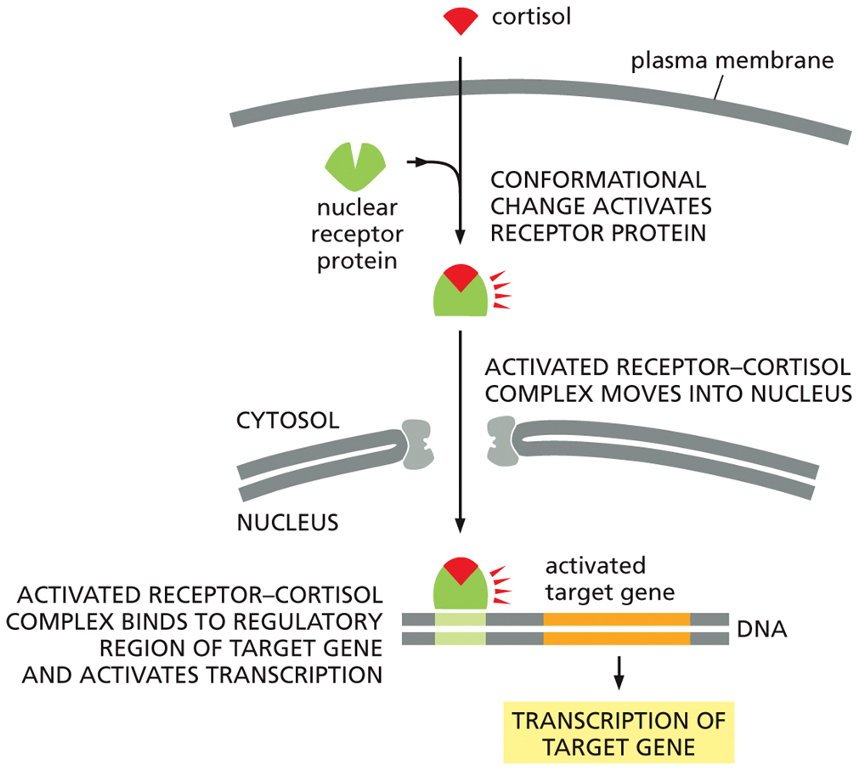
Cortisol Mechanism of Action
The Cortisol Signaling Pathway
Entry and Binding: Because cortisol is a small, hydrophobic steroid hormone, it can easily diffuse across the cell's plasma membrane into the cytosol. There, it binds to its specific nuclear receptor protein.
Activation: This binding causes the receptor to change shape (conformational change), which activates it.
Nuclear Import: The entire activated hormone-receptor complex then moves from the cytosol into the nucleus.
Gene Activation: Inside the nucleus, the complex binds directly to the regulatory region of a specific target gene on the DNA. This binding acts as a switch to activate transcription, causing the cell to produce new mRNA from that gene.
The final result of this pathway is the synthesis of new proteins that alter the cell's function. Because this process involves creating new proteins from scratch, it is considered a relatively "slow" signaling response.
Nitric Oxide & Vascular Smooth Muscle
The Signaling Cascade
This is a classic example of paracrine signaling, where a signal from one cell type (endothelial) acts on a neighboring cell type (smooth muscle).
Signal Reception (in the Endothelial Cell): The process starts when a neurotransmitter like acetylcholine binds to a receptor on an endothelial cell, the inner lining of a blood vessel.
NO Production: This binding event activates an enzyme called NO synthase (NOS), which then produces nitric oxide gas from the amino acid arginine.
Diffusion: Because NO is a small, dissolved gas, it rapidly diffuses across the membranes of the endothelial cell and into the adjacent smooth muscle cell.
Response (in the Smooth Muscle Cell): Inside the smooth muscle cell, NO binds to and activates the enzyme guanylyl cyclase. This enzyme converts GTP into the second messenger molecule cyclic GMP (cGMP). The increase in cGMP triggers the relaxation of the smooth muscle cell.
Clinical Correlation: Nitroglycerin
This pathway is the target of the drug nitroglycerin, which is used to treat angina (chest pain). Nitroglycerin is converted into nitric oxide in the body, which relaxes the coronary arteries, improves blood flow, and relieves the pain.
Types of Cell Surface Receptors
A) An ion-channel-coupled receptor opens in response to binding an extracellular signal molecule. These channels are also called transmitter-gated ion channels.
(B) When a G-protein-coupled receptor binds its extracellular signal molecule, the activated receptor signals to a trimeric G protein on the cytosolic side of the plasma membrane, which then turns on (or off) an enzyme (or an ion channel; not shown) in the same membrane.
(C) When an enzyme-coupled receptor binds its extracellular signal molecule, an enzyme activity is switched on at the other end of the receptor, inside the cell. Many enzyme-coupled receptors have their own enzyme activity (left), while others rely on an enzyme that becomes associated with the activated receptor (right).
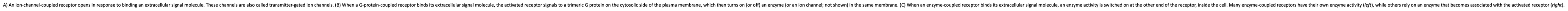
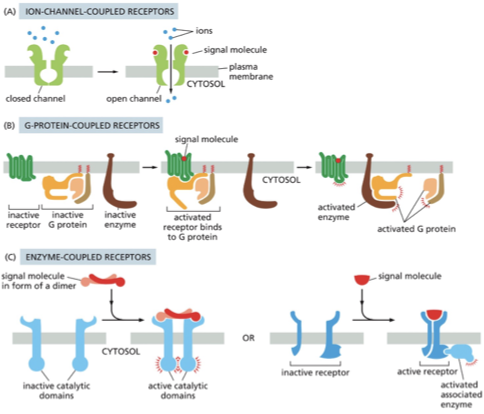
Ion Channel-Coupled Receptors
•Neurotransmitter/chemical binds to channel to causing opening
•Converts chemical signal into electrical signal
Nicotinic Receptor
•Acetylcholine molecules bind to receptor to cause opening and influx of Na+
•One site is at neuromuscular junction for skeletal muscle contraction
Myasthenia Gravis
Normal Function: In a healthy person, a nerve cell releases a chemical messenger called acetylcholine. This chemical travels across the small gap to the muscle and binds to acetylcholine receptors. As shown in the "Normal" diagram, this binding opens a channel that allows sodium ions to enter the muscle cell, triggering a muscle contraction.
Myasthenia Gravis (Blocked): In myasthenia gravis, the body's own immune system mistakenly produces antibodies that attack and block these acetylcholine receptors. The "Blocked" diagram shows these antibodies attaching to the receptors. This prevents acetylcholine from binding, so the muscle doesn't receive the signal to contract properly. This leads to the muscle weakness and fatigue characteristic of the condition.
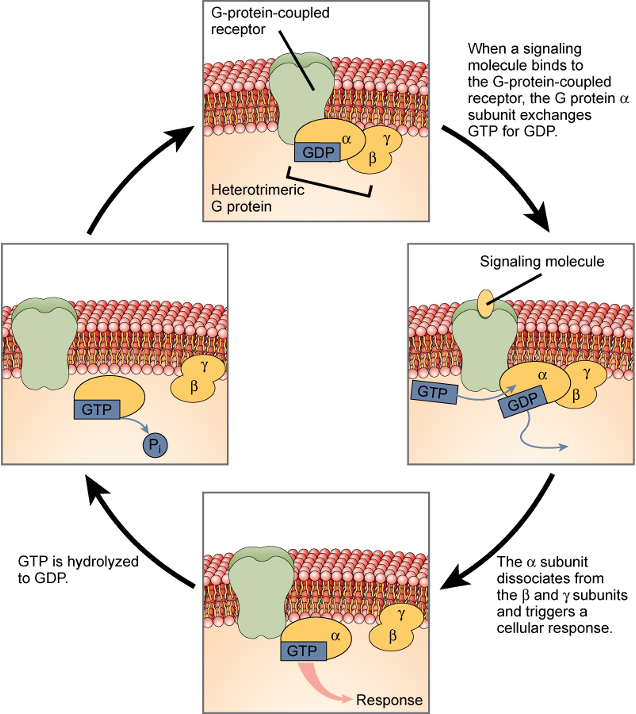
G-protein-coupled Receptors (GPCRs)
•A: unstimulated state
•receptor and G protein are inactive
•B: signal molecule
•activates receptor
•induce conformational changes of receptor and G protein
•GDP is exchanged for GTP
•trigger further conformational change that activates α and βɣ
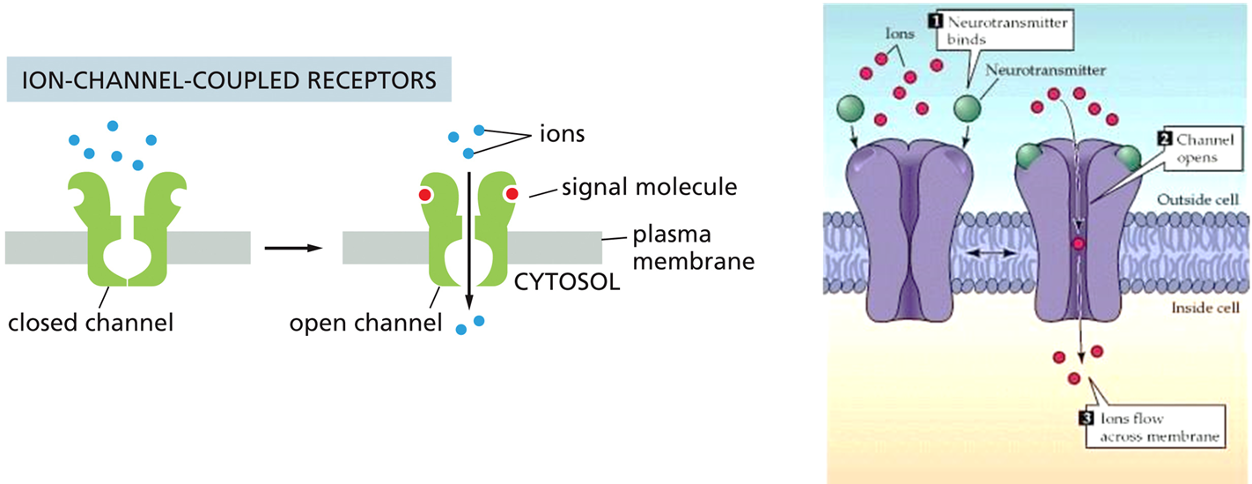
Α Subunit Switching Off
•α subunit: GTPase
•inactivate α subunit by hydrolyzing its bound GTP to GDP
•dissociate from its target protein
•re-associate with a βɣ complex
•reform an inactive G protein
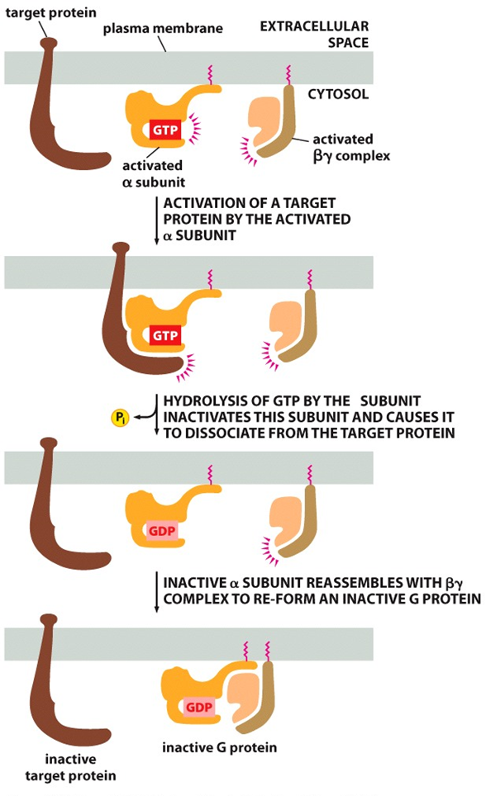
GPCR Overview
Phase 1: Activation (Flipping the Switch ON)
Signal Binds: The cycle begins when a signaling molecule (like a hormone) binds to the G-protein-coupled receptor (GPCR) from outside the cell.
The Swap: This binding activates the GPCR, causing it to interact with the nearby G protein. This prompts the α subunit of the G protein to release its bound GDP and pick up a molecule of GTP. This GDP-for-GTP exchange is the crucial "ON" switch.
Action: Now bound to GTP, the activated α subunit separates from the β and γ subunits. It is now free to move and trigger a specific cellular response by interacting with other proteins inside the cell.
Phase 2: Deactivation (The Timer Turns it OFF)
Hydrolysis: The α subunit has a built-in timer. After a short period, it hydrolyzes the GTP back to GDP by removing a phosphate group (Pi).
Reset: Once it's holding GDP again, the α subunit becomes inactive. It detaches from its target and rejoins the β and γ subunits, reforming the inactive G protein complex.
Types of G Proteins
•Effectors:
•Adenylyl cyclase
•cAMP (Gαs, Gαi)
•Phospholipase C
•DAG, IP3 (Gαq)
•Ion channels
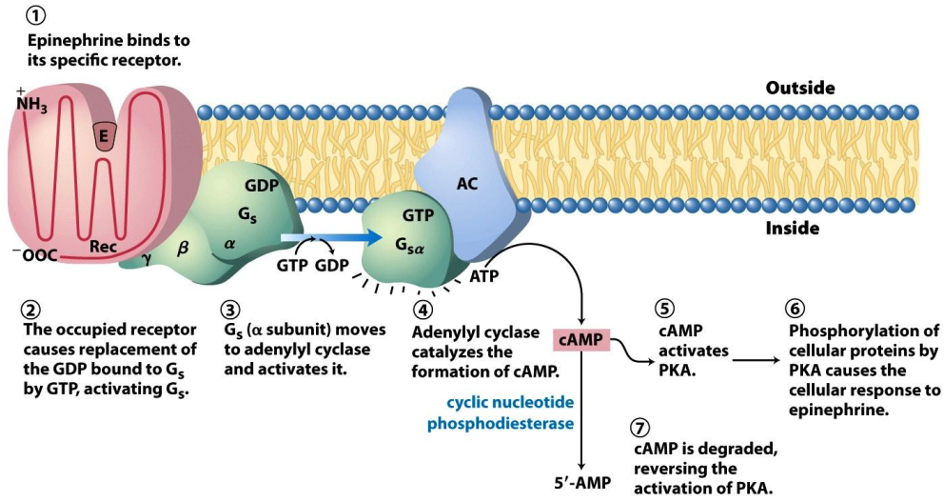
Gαs
Signal Binding: The process begins when an epinephrine molecule binds to its specific receptor protein (Rec) on the outer surface of the cell membrane.
G Protein Activation: This binding changes the receptor's shape, which in turn activates a G protein called Gs (stimulatory G protein). The activation involves the G protein's α subunit releasing a molecule of GDP and binding a molecule of GTP.
Activating the Amplifier: The activated α subunit (now called Gsα), with GTP attached, detaches and moves along the membrane until it binds to and activates an enzyme called adenylyl cyclase (AC).
Making the Second Messenger: Adenylyl cyclase is an amplifier. Once active, it rapidly converts many molecules of ATP into cyclic AMP (cAMP). cAMP is known as a second messenger because it relays the signal from the initial "first messenger" (epinephrine) throughout the cell.
Activating the Kinase: The newly produced cAMP molecules spread through the cell and bind to and activate another enzyme called Protein Kinase A (PKA).
Cellular Response: PKA is the primary effector in this pathway. It works by adding phosphate groups to other proteins (phosphorylation), which activates them. This triggers the actual cellular response to epinephrine, such as the breakdown of glycogen into glucose for energy.
Signal Termination: The signal needs a shut-off switch. An enzyme called cyclic nucleotide phosphodiesterase is always active in the cell, continuously degrading cAMP into 5'-AMP. This degradation quickly reverses the activation of PKA, ensuring the response stops once the epinephrine signal is gone.
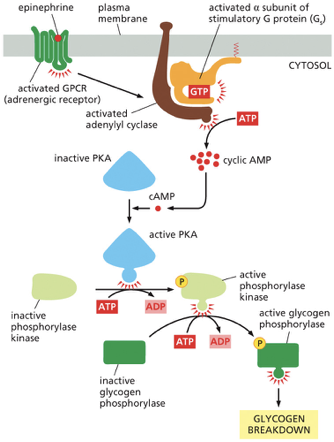
cAMP
Epinephrine Binds: The process starts when epinephrine binds to its specific receptor, an adrenergic receptor, which is a type of G protein-coupled receptor (GPCR).
G Protein Activation: This binding activates the receptor, which then activates the stimulatory G protein (Gs). The G protein's alpha subunit swaps GDP for GTP and becomes active.
Adenylyl Cyclase Activation: The activated alpha subunit of the Gs protein moves along the membrane and activates the enzyme adenylyl cyclase.
cAMP Production: Adenylyl cyclase is the first major amplification step. It rapidly converts many molecules of ATP into cyclic AMP (cAMP), a second messenger that spreads the signal throughout the cell's cytosol.
PKA Activation: The surge in cAMP activates Protein Kinase A (PKA). Four cAMP molecules bind to each inactive PKA enzyme, releasing its active subunits.
Phosphorylase Kinase Activation: This is the second amplification step. Each active PKA enzyme uses ATP to phosphorylate and activate many molecules of another enzyme, phosphorylase kinase.
Glycogen Phosphorylase Activation: This is the third and final amplification step. Each active phosphorylase kinase uses ATP to phosphorylate and activate many molecules of the enzyme glycogen phosphorylase.
Glycogen Breakdown: Active glycogen phosphorylase is the enzyme that performs the final job. It breaks down glycogen (a storage polymer of glucose) by cleaving off individual glucose units (as glucose-1-phosphate), which can then be used by the cell for quick energy.
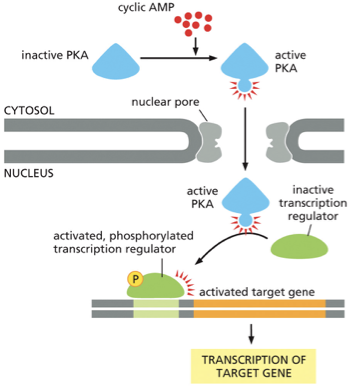
cAMP & Genes
PKA Activation in the Cytosol: The process begins when the concentration of the second messenger, cyclic AMP (cAMP), rises in the cytosol. These cAMP molecules bind to and activate Protein Kinase A (PKA).
Nuclear Entry: Once activated, PKA can travel from the cytosol into the nucleus through the nuclear pores.
Activating a Transcription Regulator: Inside the nucleus, the active PKA finds an inactive transcription regulator (also known as a transcription factor). PKA activates this regulator by adding a phosphate group to it (phosphorylation).
Gene Activation: The newly activated and phosphorylated transcription regulator binds to a specific control region on the DNA, which activates a target gene.
Transcription: This binding initiates the transcription of the target gene, where the cell's machinery reads the gene and creates a messenger RNA (mRNA) copy. This mRNA will later be used to synthesize a new protein, thus changing the cell's function.
Cholera Toxin
Toxin can:
•Modify alpha subunit & lock protein in active states
•AC is continually stimulated for increased cAMP
•Leads to chronic outflow of Cl- & H2O - diarrhea
G-Inhibitory Protein
Ligand Binding: A specific signaling molecule (ligand) binds to the receptor, activating it.
Gi Protein Activation: The activated receptor interacts with the nearby Gi protein. This causes the alpha subunit (αi) to release GDP and bind GTP, switching it to its active state.
Inhibition of Adenylyl Cyclase: The activated αi subunit separates from the βγ complex and binds to the enzyme adenylyl cyclase.
cAMP Levels Fall: Unlike the stimulatory G protein, the αi subunit inhibits adenylyl cyclase. This reduces the enzyme's activity, preventing it from converting ATP into cAMP. As a result, the concentration of cAMP inside the cell drops.
Gαq
Receptor and Gq Activation: A signal molecule binds to a G protein-coupled receptor (GPCR), activating it. The receptor then activates a specific type of G protein called Gq.
Phospholipase C Activation: The activated Gq protein (specifically its alpha subunit) then activates an enzyme embedded in the membrane called phospholipase C.
Creation of Two Second Messengers: Phospholipase C acts on a specific membrane lipid called an inositol phospholipid. It cleaves this lipid into two separate molecules, which both act as second messengers:
Inositol 1,4,5-trisphosphate (IP₃): A small, water-soluble molecule that detaches from the membrane and diffuses into the cytosol.
Diacylglycerol (DAG): A lipid molecule that remains embedded in the plasma membrane.
IP₃ and Calcium Release: The IP₃ molecule travels to the endoplasmic reticulum (ER) and binds to a special IP₃-gated calcium (Ca2+) channel. This opens the channel, causing a rapid release of stored Ca2+ ions from the ER lumen into the cytosol. This sudden spike in cytosolic calcium is itself a widespread intracellular signal.
DAG and PKC Activation: The DAG that remains in the membrane, along with the increased cytosolic Ca2+ concentration, works to recruit and activate another enzyme called Protein Kinase C (PKC). The "C" stands for calcium, as its activation is dependent on it. The activated PKC can then phosphorylate a variety of cellular proteins, leading to a specific cellular response.
Rise in Ca2+ Triggers Biological Events
•A surge in the cytosolic concentration of free calcium is triggered by many kinds of cell stimuli
•Example: fertilization of an egg by sperm triggers increased cytosolic calcium levels in the egg
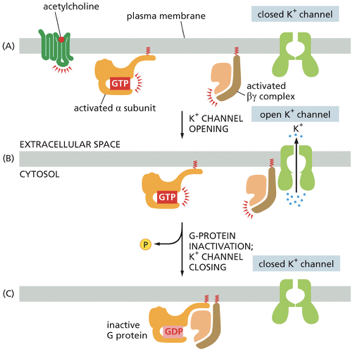
G Proteins & Ion Channels
(A) Activation
The neurotransmitter acetylcholine binds to its specific G protein-coupled receptor (GPCR).
This activates the associated G protein, causing the α subunit to exchange its GDP for GTP.
The G protein then splits into two active parts: the activated α subunit and the activated βγ complex.
(B) Channel Opening
In this particular pathway, it's the activated βγ complex that acts as the signaling molecule.
It diffuses a short distance along the membrane and binds directly to a closed K+ channel.
This binding forces the channel to open, allowing positively charged potassium ions to flow out of the cell, down their concentration gradient. This outflow of positive charge makes the cell membrane more polarized (hyperpolarized), which in the case of heart muscle, makes it harder to excite and thus slows the heart rate. ❤🩹
(C) Inactivation and Closing
The system has a built-in timer. The α subunit hydrolyzes its bound GTP back to GDP, which inactivates it.
The now-inactive α subunit re-binds to the βγ complex, reforming the complete, inactive G protein.
With the βγ complex no longer available to bind to the channel, the K+ channel closes, and the system returns to its resting state, ready for another signal.
Enzyme-Coupled Receptors
•Spans membrane once
•Binding of signal causes dimerization
•Result? Activation of kinase domains on receptor or receptor-associated enzymes
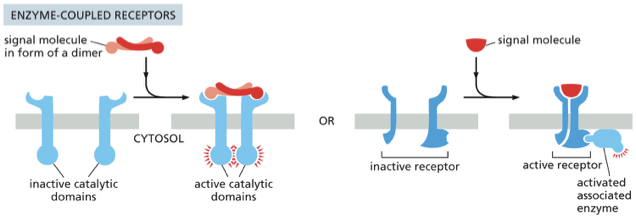
Receptor Tyrosine Kinases (RTKs)
Dimerization: The process begins when a signal molecule, typically in the form of a dimer, binds to two inactive RTK molecules, pulling them together to form a stable pair (a dimer) in the cell membrane.
Kinase Activation: This dimerization pushes the two tyrosine kinase domains of the receptors close to each other. This proximity stimulates their kinase activity, essentially "waking up" their enzymatic function.
Autophosphorylation: The activated kinase domains then phosphorylate each other on multiple tyrosine residues. This process is called autophosphorylation or cross-phosphorylation. Each receptor in the pair adds phosphate groups (P) to its partner.
Recruitment of Signaling Proteins: The newly phosphorylated tyrosines act as high-affinity docking sites for a variety of intracellular signaling proteins. Different proteins recognize and bind to specific phosphorylated sites.
Signal Relay: Once bound to the receptor, these signaling proteins become activated. They can then relay the signal deeper into the cell, triggering multiple downstream intracellular signaling pathways and ultimately leading to a change in cell behavior.
Ras & RTKs
•Receptor activated – adaptor protein docks to phosphotyrosine
•Adaptor recruits Ras guanine nucleotide exchange factor (Ras-GEF)
•Ras-GEF stimulates Ras protein (GDP exchanged for GTP)
•Ras protein stimulates downstream signaling
•E.g., involved in cell growth, differentiation, & survival
When Ras and Ras-related proteins are dysregulated?
Metastasis and lack of apoptosis
Mutated Ras in cancer
•Mutations inactivates GTPase activity of Ras
•Ras could not shut itself off and promote uncontrolled cell proliferation
•~30% of human cancers contain Ras mutation