Molecular Compound Shapes
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
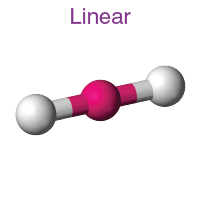
1
New cards
Linear
Hybridization: sp
Bond Angle: 180o
Example: HCI, CO2
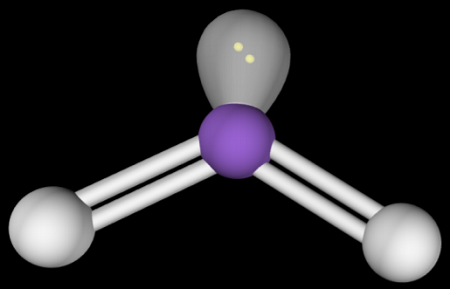
2
New cards
Bent (1 Lone Pair)
Hybridization: sp2
Bond Angle: <120o
Example: O3, SO2
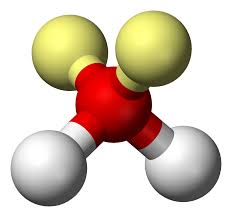
3
New cards
Bent (2 Lone Pairs)
Hybridization: sp3
Bond Angle: 104.5o
Example: H2O, SBr2
4
New cards
Trigonal Planar
Hybridization: sp2
Bond Angle: 120o
Example: SO3, BF3
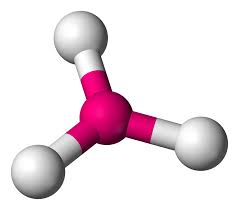
5
New cards
Tetrahedral
Hybridization: sp3
Bond Angle: 109.5o
Example: CH4, SIF4
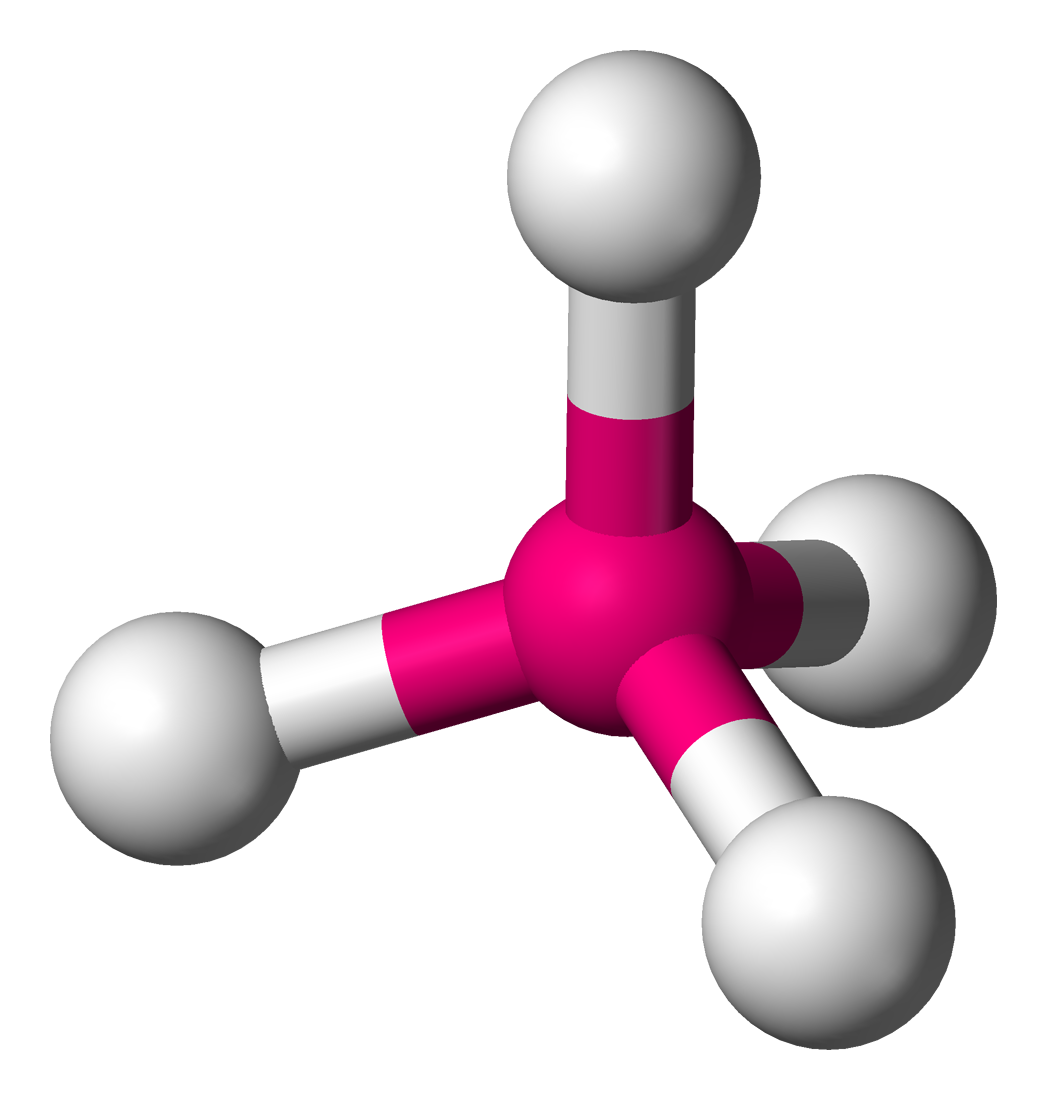
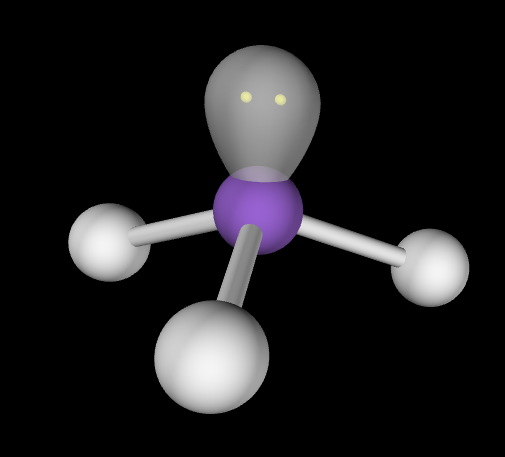
6
New cards
Trigonal Pyramidal
Hybridization: sp3
Bond Angle: 107o
Example: NH3, PCl3
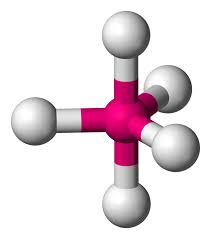
7
New cards
Trigonal bipyramidal
Hybridization: sp3d
Bond Angle: 120o & 90o
Example: PCl5
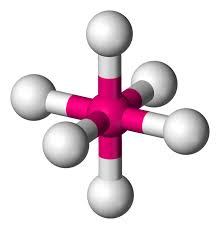
8
New cards
Octahedral
Hybridization: sp3d2
Bond Angle: 90o
Example: SF6