Thermodynamics
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
Define enthalpy change
Heat energy change at a constant pressure
Define lattice formation enthalpy
The enthalpy change when 1 mole of a solid ionic compound is formed from its gaseous ions
E.g. Na+ (g) + Cl- (g) →NaCl (s)
Is lattice formation enthalpy exothermic or endothermic - explain why?
Exothermic
In the ionic compound formed new attractions between the ions are being formed
Energy released when attractions formed
Define lattice dissociation enthalpy
Enthalpy change when 1 mole of a solid ionic compound is completely dissociated into its gaseous ions.
E.g. NaCl (s) → Na+ (g) + Cl- (g)
Is lattice dissociation enthalpy exo or endo - explain why?
Endothermic
Breaking attractions requires energy
What does the value of lattice enthalpies depend on?
Strength of electrostatic attraction between ions
What does the strength of electrostatic attraction depend on and explain?
Size of ion→ Smaller the radius of the ion, stronger the attraction (smaller ions can pack closely together in ionic lattice)
Magnitude of charge→ Larger charge, stronger the attraction
(Basically it depends on charge density)
Define charge density
How concentrated the charge is in an ion
The higher the charge density→ Stronger attraction between ions
More exothermic the lattice formation enthalpy
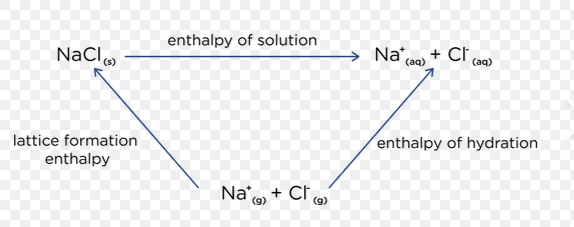
What is required to dissolve an ionic compound?
Where does it come from
And what proceeds to happen
Energy required to break apart the lattice (Lattice dissociation enthalpy)
Energy comes from the water (solvent)
Water forms new attractions to the ions in the lattice→ Creating an aqueous solution
Ions dissolved until there is infinite dilution meaning no electrostatic attraction between + and - ion, attraction is now between ions and water solvent.
Define enthalpy of hydration
Enthalpy change when 1 mole of gaseous ions become aqueous ions
E.g. Na+ (g)→ Na+ (aq)
Exothermic
Define enthalpy of solution
Enthalpy change when 1 mole of an ionic solid dissolves in enough solvent to form an infinitely dilute solution
NaCl (s) + aq → Na+ (aq) + Cl- (aq)
What does the enthalpy of solution depend on?
Balance between the lattice dissociation enthalpy which is endothermic and hydration enthalpy which is exothermic
If magnitude of hydration enthalpy> lattice dissociation enthalpy → the enthalpy of solution will be exothermic / vice versa
Hess cycle for enthalpy of solution
Switch direction of arrow for lattice dissociation enthalpy
Hoe does the Born Haber cycle work
Works in the same way as Hess cycle
Enthalpy change is still independent to route taken
Born Haber cycles have energy as a y axis
Arrow pointing up = Endothermic
Arrow pointing down= Exothermic
What is lattice enthalpy
A measure of attraction between ions
(Cannot be measured directly in an experiment)
What is theoretical lattice enthalpy?
Lattice enthalpy value you calculate using a model that assumes ions are perfect spheres and considers size, charge and arrangement of ions in the lattice.
What is experimental lattice enthalpy?
Carry out a series of experiments
Construct a Born-Haber cycle
To workout lattice enthalpy
What is enthalpy of formation?
Enthalpy change when 1 mole of a compound is formed from its elements in their standard states.
(In BH cycle its 1 mole of an ionic compound)
Exothermic
E.g. Na (S) + ½ Cl2 → NaCl (S)
What is first ionisation energy?
Energy required to remove 1 mole of electrons from 1 mole of gaseous atoms to form 1 mole of gaseous ions with a +1 charge.
Endothermic
E.g. Mg (g) → Mg+ (g) + e-
What is second ionisation energy?
Energy required to remove 1 mole of electrons from 1 mole of gaseous 1+ ions to produce one mole of gaseous 2+ ions
Endothermic
E.g. Mg+ (g) → Mg2+ (g) + e-
What is first electron affinity?
Enthalpy change when 1 mole of gaseous atoms gain 1 mole of electrons to form 1 mole of gaseous ions with a -1 charge
Exothermic (New attraction formed between the electron and nucleus of atom)
E.g. o (g) + e- → o- (g)
What is second electron affinity?
Enthalpy change when 1 mole of gaseous 1- ions gain one electron per ion to produce gaseous 2- ions
Endothermic ( The o- and e- repel each other - energy supplied/needed to overcome that repulsion)
E.g. o- (g) + e- → o2- (g)
What is atomisation (for an element)
Enthalpy change when 1 mole of gaseous atoms is formed from the element in its standard state.
Endothermic
E.g. Na (s) → Na (g)
Example of atomisation for a diatomic element ?
½ Cl2 (g) → Cl (g)
( Half the moles of Cl2 has the same number of Cl atoms as 1 mole of Cl (g) )
What is bond disassociation enthalpy
Standard molar enthalpy change when one mole of a covalent bond is broken into 2 gaseous atoms
E.g. Cl2 (g) → 2Cl (g)
What is atomisation ( For a compound)
Enthalpy change when 1 mole of a compound in standard state is converted into gaseous atoms
E.g. NaCl (s) → Na (g) + Cl (g)
Enthalpy change of formation = sum of all enthalpy changes when..
Born Haber cycle includes lattice formation enthalpy
General format of Born Haber cycle
1) Enthalpy change of formation
2) Atomisation of x
3) Atomisation of y
4) F.I.E of x
5) Electron affinity of y
6) Lattice formation enthalpy
What is a flaw of theoretical lattice enthalpy?
Makes the assumption that ions are perfectly spherical
But ions don’t exist as isolated perfect spheres
Instead electron clouds around ions are attracted towards each ion
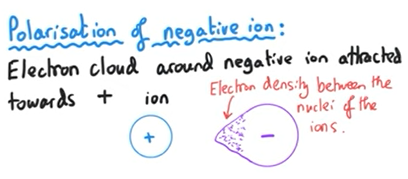
What is meant by polarisation of negative ion?
Electron cloud around negative ion is attracted towards positive ion
So there is electron density between the nuclei of the ions
What effects the polarisation of a negative ion?
Higher charge density of the positive ion →More polarisation of negative ion
A large singly charged negative ion will be more polarisable→ Electron cloud is further away from nucleus so it is more easily distorted by positive ion → A singly charged ion has more electrons than protons so the nucleus cannot hold them as tightly. (But in a small singly charged negative ion the cloud is closer to nucleus so less polarisable)
(Electron cloud refers to all electrons but the outermost electrons are mainly involved in polarisation)
What does the experimental lattice enthalpy value reflect?
Polarisation of negative ions
Lattice has covalent character (if there a higher degree of polarisation)
Why does a lattice have covalent character in experimental lattice enthalpy value?
Because of the electron density between the nuclei of the 2 ions during polarisation
(Basically what happens in covalent bond - i.e shared electron attracted to nuclei of both atoms)
So ions are held more tightly together
And the experimental lattice enthalpy (of formation) is more exothermic
-Check notes for additional guidance
Chemical reactions that happen spontaneously are …
Feasible reactions
When does a reaction occur and how does this happen?
When it is overwhelmingly probable by chance alone
Happens because a chemical system will change from one where there is limited ways of arranging energy to a system with more ways of arranging energy.-Occurs by random changes
Trend is from order to disorder
(When we consider whether or not a reaction is going to be feasible we are basically asking whether this will result in an increase in disorder)
Why are exothermic reactions more likely to be feasible?
Heat energy is released to surrounding gas molecules
Warmer gas molecules→ have more kinetic energy→ move around more→ become more disordered.
Define entropy
Entropy of a chemical system is a measure of the energy that a system has at a particular temp, per mole of each chemical
Units of entropy (s)
Joules per kelvin per mole
J K -1 mol-1
Which way do feasible reactions go spontaneously?
In the direction of increasing disorder i.e increasing entropy
What does an increase in moles mean for entropy?
More moles of a chemical→ more disordered than a few moles
Increase in moles→ Increase in entropy
E.g. 1mole (of reactants) → 2moles of products : Increasing entropy i.e positive entropy change
2moles → 1 mole : Decreasing entropy, negative entropy change (This reaction is less likely to be feasible as there’s a decrease in disorder)
Equation to calculate entropy change

What is Gibbs Free- energy change ∆G
Balance between entropy and enthalpy in a system
The balance determines feasibility of a reaction
(Reaction will only be feasible if there is an overall movement of “free-energy” [all types of energy] out of the chemical system to the surrounding)
Gibbs free energy equation and units

A reaction is feasible at a specific temperature if ..
Gibbs free energy change is equal to or below zero (negative)
∆G is more likely to be negative and the reaction feasible if..
∆H is negative ( Exothermic reaction)
∆S is positive (Increased disorder)
When is a reaction never feasible?
When ∆S is negative and when ∆H is positive (endo)
If a reaction has -∆H (exo) and -∆S (decreasing disorder)..
the reaction will be feasible if ∆H> T∆S
it gets less feasible as temp increases because enthalpy value is what will cause this reaction to be feasible so its size needs to be greater.
If a reaction has +∆S and +∆H..
Reaction will be feasible if T∆S>∆H
So as temp increases this reaction will get more feasible.
Why may a reaction not always be spontaneous even if it is feasible?
∆G can be negative → making reaction feasible but activation energy must be very high
so even if ∆G is negative, reaction may not be spontaneous if Ea. is very high
What temperature does a reaction become feasible?
This temperature is the point where the reaction goes from being infeasible to feasible (∆G= 0)
So when asked to workout the temperature for when the reaction becomes feasible assume ∆G is 0
Rearrange Gibbs free energy equation to find temperature
T = ∆H / ∆S
Before substituting in entropy change, you must convert it into KJ K-1 Mol-1
A situation where ∆G = 0 and what this allows you to do?
A change of state
This is because at MP (S→L) and BP (L→G) forward and reverse reactions occur at the same rate.(Dynamic eqm)
Can use Gibbs free energy equation to workout out at what temp MP or BP should occur
Gibbs free energy equation into straight line equation
∆G = -∆ST +∆H
Y = m x + c
Gradient is - ∆S
Draw and explain a free energy graph with a positive gradient
Refer to notes.
Draw and explain a free energy graph with a negative gradient
refer to notes
Draw a graph for a reaction that is never feasible and one where the reaction is always feasible
Refer to notes
More negative a value for ∆G …
More feasible the reaction
More likely that the reaction goes to completion (almost all of the reactants are converted into products)
More positive a value for for ∆G …
The less likely the reaction will go to completion
Reverse reaction is more feasible
( If the ∆G value for the forward reaction is +250 the value for the reverse reaction will be -250)