Exam #3 - Adv Clinical Chemistry
1/95
Earn XP
Description and Tags
Ch. 14, 16-17, 24-25
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
96 Terms
2 pituitary hormones that are necessary for life & the rest “just for fun” (6)
TSH, ACTH
“fun”: hGH, LH, FSH, PRL, ADH, oxytocin
Hypothalamic releasing factors (5)
Corticotropin Releasing Factor (CRF)
Thyrotropin-releasing hormone (TRH)
Growth hormone-releasing hormone (GHRH)
Somatostatin
Gonadotrophin-releasing hormone (GnRH)
Anterior pituitary hormones (6)
TSH = Thyroid Stimulating Hormone
ACTH = Adrenocorticotropic Hormone
hGH = human Growth Hormone
LH = Luteinizing hormone
FSH = Follicle Stimulating Hormone
PRL = Prolactin
Posterior pituitary hormones (2) & Pituitary trophic hormones (function)
oxytocin & antidiuretic hormone (Arginine Vasopressin)
“feed” and increase size of target cell in end-organs to maintain their normal size, mass, and function
Negative feedback loop (analogy)
works like thermistor in house thermometer: control output of a furnace
analogy: hormone level is like temp; warm = high circulating, cold = low circulating
pituitary secreting hormone is like furnace generating heat; furnace turns on when cold & off when warm
pituitary turns on (secrete stimulating hormone) when temp is cold
pituitary turns off secretion when temp is warm
temp in well controlled room fluctuates with time as a sine curve
General rules of endocrinology practice (6)
A gland is not secreting hormone when it should → kick start it
A gland is secreting too much hormone → suppress it
Stimulation & Suppression Tests are used in the setting of thyroid & adrenal endocrinopathies
Primary disease: problem is confined within gland
Secondary disease: problem is in pituitary function
Tertiary disease: problem in hypothalamic function
Hypothalamus & Pituitary: Circulation (input, anterior & posterior)
master integrator of input from higher CNS
input: pain, stress, cold, light/dark cycles
hypothalamic-hypophyseal portal circulation collects blood from capillaries of hypothalamus → plexus of veins surrounding pituitary stalk → directs blood into anterior pituitary gland
hypothalamic hormones are carried down from neurons of Supraoptic & PV Nuclei → directly into posterior pituitary
Hypothalamic releasing factor: Releasing location, measure, CRF, TRH
not released directly into peripheral circulation; released into a portal circulation that supplies anterior pituitary
released directly by neurons into posterior pituitary (PP)
Difficultly measured as blood draining hypothalamus must be sampled in Inferior Petrosal Sinus (invasive)
CRF: stimulates the release of ACTH by corticotrophs in anterior pituitary (AP)
TRH: stimulates release of TSH by thyrotrophs in AP
Disorders of pituitary (2)
Trauma/injury/radiation usually causes hypopituitarism: panhypopituitarism involves loss of stimulatory hormones from the AP & PP
Adenomas:
Non-functioning: do not secrete hormones
Functioning: usually secrete GH & PRL, rarely TSH, ACTH, FSH, LH from the anterior pituitary
Thyroid function: Specialization, feedback loop players, follicular cells, protein bound
specialized for endocrine hormone production
feedback loop: pituitary thyroid stimulating hormone (TSH/thryotropin) & triiodothyronine (T3)
follicular cells (FC) express receptor for TSH → promotes thyrocytes’ growth & biosynthetic functions
thyroid hormones extensively protein bound (99.9%): thyroid binding globulin (TBC) & Albumin; free thyroid hormone is important to feedback loops & disease
Thyroid function (FC, Clcitonin, thyroglobulin, iodinases, C cells)
FC produce thyroxine (T4) & smaller amounts of triiodothyronine (T3)
calcitonin: tumor marker of medullary thyroid cancer
thyroglobulin: post-treatment tumor marker of residual thyroid cancer
extrathyroidal T4 converted to T3 by peripheral iodinases
T3 binds to nuclear receptor regulating + / - genes
thyroid contains parafollicular/C cells: produce calcitonin (inhibits bone resorption)
Life-sustaining action of thyroid hormones (development, regulation, modulation, FC)
fetal & childhood growth & CNS development
regulate HR, myocardial contraction & relaxation, GI motility, renal clearance, weight & lipid metabolism
modulation of BMR, energy expenditure, O2 consumption, heat generation, metabolism
FC: synthesize hormonal precursor protein thyroglobulin (TG) & concentrate iodide intracellular from circulation
Dietary iodine intake (adults, pregnant women, deficiency, <50)
150 μg for adults, 200 μg for pregnant and lactating women, & 90 μg for children
the kidneys excrete most iodide
urinary iodide excretion: index of dietary intake
Dietary iodine deficiency: daily iodine intake < 100 μg/d
iodide intake < 50 μg/d: normal-sized thyroid cannot sustain adequate hormone production → gland enlargement (goiter) & hypothyroidism
Thyroid hormone synthesis (symporter, use of iodine, uptake, requirements, other tissues)
Thyrocytes express Na-iodide symporter (spans the cells’ basal membranes and actively transports iodide from the blood)
thyroid gland uses only a fraction of the iodide supplied to it for hormone synthesis, the remainder returns to the extracellular fluid pool.
normal fractional uptake of iodide is approximately 10-30% after 24 hours
Thyroid hormone synthesis requires: NIS, TG, & enzyme thyroid peroxidase (TPO) all be present, functional, and uninhibited
Salivary, gastric, & breast tissues: express NIS & concentrate iodide less than thyroid, but these tissues do not organify or store iodide
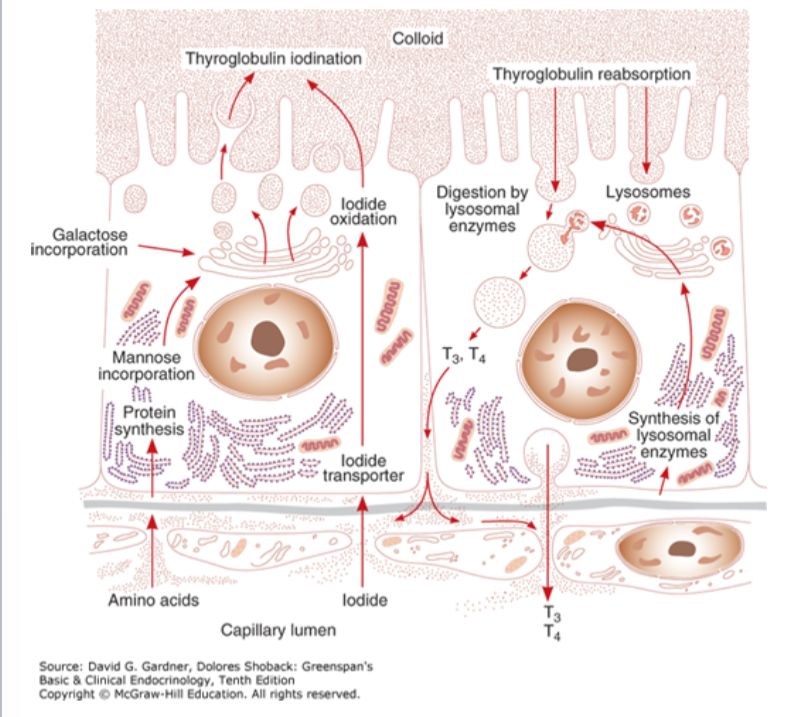
Thyroid hormone synthesis with iodide steps (6)
Iodide trapping: Active transport of iodide across the basement membrane into the thyroid cell with Na+
Oxidation of iodide (TPO) & iodination of tyrosyl residues in TG
Coupling: Linking pairs of iodotyrosine molecules within TG → form iodothyronines T3 and T4
Pinocytosis & then proteolysis of TG with release of free iodothyronines & iodotyrosines into the circulation
Deiodination of iodotyrosines within the thyroid cell (with conservation & reuse of the liberated iodide)
Intrathyroidal 5′-deiodination of T4 to T3
TPO: Function, TSH, drug inhibitors)
membrane-bound glycoprotein (MW 102 kD) containing a heme moiety: catalyzes iodide oxidation & covalent linkage of iodine to the tyrosine residues of TG
TPO gene expression is stimulated by TSH
The thiocarbamide drugs (methimazole, carbimazole, and propylthiouracil (PTU)) are competitive inhibitors of TPO
Resulting ability to block thyroid hormone synthesis useful in treatment of hyperthyroidism
Thyroid hormones: T3 & T4 Transport (free vs bound, factors affecting TBG levels)
T3 & T4 are extensively protein bound by albumin or TGB
0.01% free and biologically active with thyroid hormone nuclear receptors & feedback loops
pregnancy, estrogen-secreting tumors, & estrogen therapy all increase sailic acid content of TBG molecule → decreased metabolic clearance & elevated serum TBG levels
Factors that decrease TBG levels
nephrotic syndrome & protein-losing enteropathy
liver failure
major systemic illness due to cleavage by leukocyte proteases & reduction in TBG’s binding affinity for thyroid hormones
both lower serum total thyroid hormone
Thyroid hormones: TRH Feedback loop (location, gene expression, AP, related to TSH)
TRH found: other portions of hypothalamus, brain, spinal cord (functions as neurotransmitter)
TRH gene expression is (-) regulated by thyroid hormone (T3 delivered by circulation & arising from T4 deiodination in peptrigergic neurons)
Anterior pituitary: TRH binds to specific membrane receptor located on thyrotropes → stimulates synthesis & release of TSH
TRH-stimulated TSH secretion is pulsatile
Thyroid hormones: TSH (structure)
28-kD glycoprotein composed of alpha & beta subunits that are noncovalently linked
alpha subunit: common to 2 pituitary glycoproteins, FSH, LH, & placental hCG
beta subunit: unique for each glycoprotein hormone, conferring specific binding properties & bio activity
TSH: Controls, stimulates, intact, detection
TSH controls thyroid cell growth & hormone production by binding to specific TSH receptor
TSH stimulates: changes in thyroid cell morph, growth, iodine metabolism, O2 uptake glucose oxidation in thyrocytes, & synthesis and secrete thyroid hormones
intact TSH & the isolated alpha subunit both present in circulating blood
TSH detectable by immunoassay in concentrations: 0.5-4 mU/L & 0.5-2 microgram/L
TSH levels: Primary hypothyroidism, thyrotoxicosis, half-life, high/low T3/T4
serum TSH level is increased in primary hypothyroidism & decreased in thyrotoxicosis (endogenous or excessive oral intake of thyroid hormones)
plasma half-life of TSH: 30 minutes
TSH synthesis & release inhibited by high serum levels of T3 & T4
TSH synthesis & release stimulated by low levels of thyroid hormone
TSH: 2 factors & Thyrotoxicosis on synthesis & release
2 major factors that control synthesis & release of TSH:
T3 level within tyrotroph cells (regulate mRNA expression, TSH translation, hormone’s release)
TRH (control posttransitional glycosylation & release)
thyrotoxicosis (hyperthyroidism) can suppress serum TSH levels beneath limits of assay detection & recovery of normal TSH secretion
may require wks or months after restoration of normal thyroid hormone levels
Thyroid hormones: Genomics (2 mechanisms, TRs to TREs, TRs other function)
thyroid hormones exert actions through 2 mechanisms:
genomic actions effected through T3 interactions with nuclear receptors, regulate gene activity
nongenomic actions mediated by T3 & T4 interactions with enzymes, glucose transporters, nitrochontrial, & membrane proteins
TRs bind to TREs (typically paired), specific oligonucleotide sequences
TRs also function as heterodimers with receptors for other transcription factors (such as retinoid X & retinoic acid receptors)
Thyroid hormones: Genomics (TREs location, + regulated genes, TRE bound)
TREs generally located upstream of transcription start site for coding regions of thyroid hormone (responsive genes)
in (+) regulated genes: unbound TRs interact with corepressors & silence mediator for retinoic & thyroid hormone receptors (SMRT) → repress basal transcription by recruiting histone deacetylases that alter nearby chromatin structure
when TRs bound by T3: corepressor complexes released & T3-bound TRs associate with coactivator complexes (promote local histone acetylation)
Thyroid hormones: Physiology (transcriptional effects, 12)
transcriptional effects of T3 have a lag time of hours or days to achieve full effect
fetal development: brain development & skeletal maturation
O2 consumption & free radical formation
heat production: increased basal metabolic rate, Na/K ATPase activity (heat/cold intolerance in thyroid disease)
Cardiovascular: increase in cardiac output, contractility, sensitivity to epinephrine
Sympathetic nervous system: increased adrenergic sensitivity
Pulmonary: hypoventilation in hypothyroidism; weakened respiratory muscles in hyperthyroidism
Hematopoietic: potentiates activity of EPO; increases 2,3-BPG
Gastrointestinal: gut motility
Skeletal: bone turnover
Neuromuscular Effects
Lipid and Carbohydrate Metabolism: cholesterol synthesis and degradation
Endocrine: growth, development, puberty
Thyroid: Testing guide (screening, 1 change, free levels)
screen for thyroid disease begins with TSH & total/free T4 levels
1 fold change in thyroid hormone → several fold changes in TSH
free hormone levels are important especially in pregnancy (high TBG) & liver disease (low TBG)
RR: Serum T4, free T4, serum T3, free T3, serum thyrotropin, thyroxine-binding globulin
serum T4: 4.6-12 ug/dL
free thyroxine (FT4): 0.7-1.9 ng/dL
serum T3: 80-180 ng/dL
free T3 (FT3): 230-619 pg/dL
serum TSH: 0.5-6 uU/mL
TBG: 12-20 ug/dL T4 + 1.8 ugm
Hypothyroidism: Classifications & most common cause
Primary (most common), Secondary (pituitary TSH deficiency), Tertiary (hypothalamic TRH deficiency), Peripheral thyroid hormone resistance
Most common cause: autoimmunity
chronic lymphocytic thyroiditis (Hashimoto’s)
Autobody panels: serum anti-TPO, blocking TSI (Grave’s/Perry) or less sensitive anti-TG
Hyperthyroidism/Thryotoxicosis (thyrotoxic crisis, ademona, goiter, treatment)
thyrotoxic crisis (thyroid storm): acute exacerbation of all the symptoms & signs of thyrotoxicosis, often as a syndrome that may be life-threatening
toxic adenoma: functioning adenoma hypersecreting T3 & T4
Toxic multinodular Goiter (Plummer disease)
treatment: methimazole (TPO inhibitor) surgery, radioactive iodine, propanolol & other beta blockers
Grave’s disease (what, disorder, ophthalmopathy, treatment)
Primary hyperthyroidism: most common form of thyrotoxicosis (females 5x more likely)
autoimmune disorder associated with TSIs: thyroid stim immunoglobulins (autoantibodies) that activate TSH receptor → oversecretion of T4 & T3
thyroid -associated Ophthalmopathy: immune system (targeting the TSH receptor) attacks tissues around the eyes → releases cytokines that cause orbital tissues to swell & expand—through increased fat and muscle size—which pushes the eyeballs forward
treatment: methizamole (TPO inhibitor) surgery, radioactive iodine, propanolol & other beta blockers
Thyroid testing guide: Classical presentation (hypothyroidism, hyperthyroidism, goiter, autoantibodies)
hypothyroidism: fatigue, cold intolerance, weight gain, constipation, dry skin, myalgia, menstrual irregularities, bradycardia, hypertension, delayed relaxation phase of deep tendon reflexes (myxedema coma)
Hyperthyroidism: anxiety, emotional lability, weakness, tremor, palpitations, heat intolerance, increased perspiration, & weight loss despite a normal or increased appetite (thyroid storm/thyrotoxicosis)
Goiter: caused by prolonged elevated TSH levels
Most thyroid disease is caused by autoantibodies, causing either immune thyroiditis (Hashimoto’s) or Graves’ disease (thyroid stimulating immunoglobulins)
Thyroid testing guide: Classical presentation (hypothyroidism, hyperthyroidism, subclinical hypothyroidism)
hypothyroidism: elevated TSH, low free T4; most patients with Hashimoto’s show elevated TPO (thyroid peroxidase) autoantibodies
hyperthyroidism: patients with primary hyperthyroidism have low TSH; most with overtly hyperthyroidism (<0.5 mU/L)
TSH levels suppressed by intact feedback loop in elevated thyroid hormone levels
diagnosis confirm by radioiodine uptake
Subclinical hypothyroidism: elevated TSH, normal T4; pituitary working overtime to maintain thyroid hormone levels
Thyroid cancer (routine tests, biopsy, prevalence, thyroglobulin, thyrogen stim, calcitonin)
routine lab tests not reliable alone → radioiodine uptake with ultrasonography & other imaging techniques
biopsy: histology (fine needle aspiration)
cancer has higher than avg prevalence in Los Almost & Rio Arriba counties
thyroglobulin: tumor marker for follow up treatment for recurring thyroid cancer
thyrogen stim test: instead of withdrawal, synthetic TSH can be given to stim thyroid & unmask residual cancer
Calcitonin: tumor marker for medullary TCa
Glucocorticoids & adrenal androgens (adrenal cortex, immunomodulatory, feedback loop)
adrenal cortex produces cortisol, aldosterone, & adrenal androgens
suppressing inflammation and immunity in the short term while potentially contributing to immune dysregulation with chronic stress
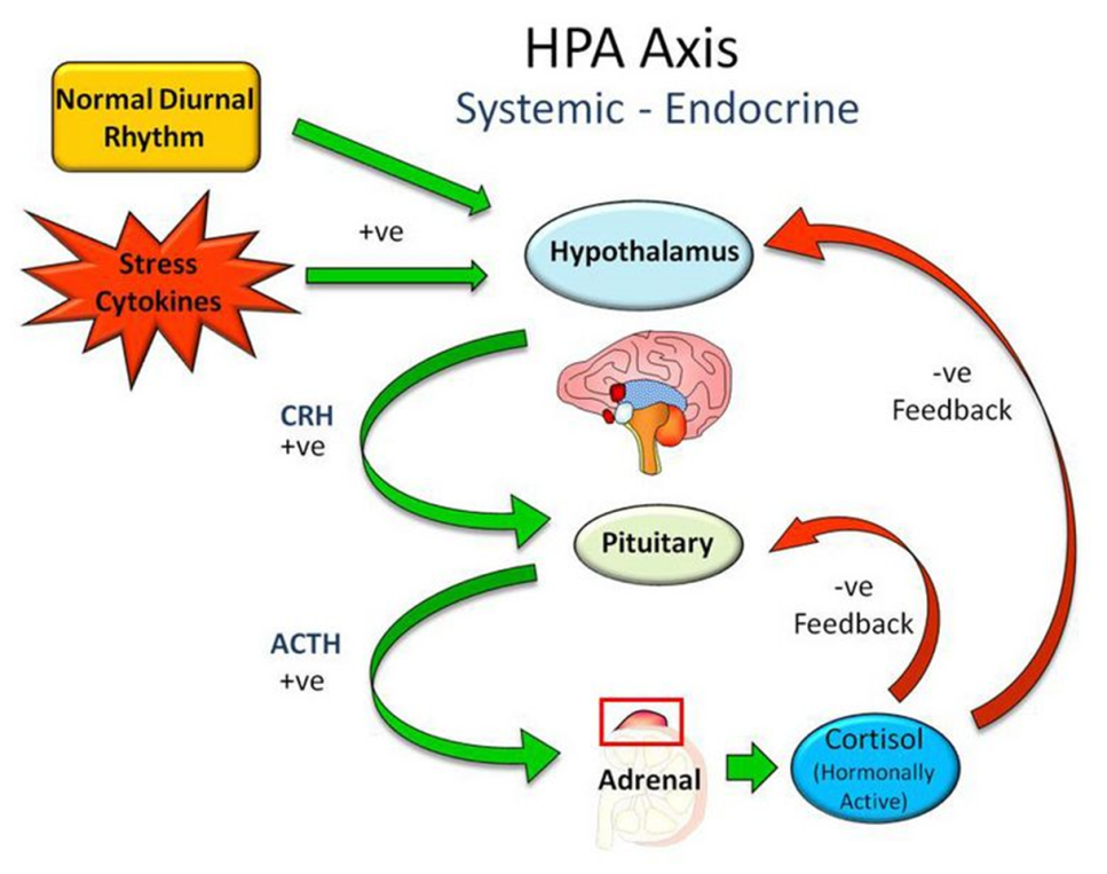
feedback loop involves ACTH (pituitary) & cortisol
Hypothalamus → CRH → Pituitary.
Pituitary → ACTH → Adrenal Glands.
Adrenal Glands → Cortisol is released into the blood
High Cortisol feeds back to SHUT OFF the Pituitary (ACTH) & Brain (CRH).
When cortisol drops, the loop starts again
Glucocorticoids & adrenal androgens: ACTH, protein-bound, salivary
ACTH secreted as part of preopiomelanocortin (POMC) precursor with melanocyte stimulating hormones
Cortisol is extensively protein bound (CBG)
Salivary cortisol is best estimate of free cortisol level
Glucocorticoids & Adrenal androgens: Cortisol overproduction, Primary Adrenal insufficiency, Congenital adrenal hyperplasia
Cortisol overproduction: Cushing’s syndrome (from any cause including stress)/Cushing’s Disease (from pituitary ACTH)
Primary adrenal insufficiency: Addison’s disease
Congenital adrenal hyperplasia: 21-hydroxylase deficiency
Biosynthesis of Adrenal androgens & Cortisol: Where, conversion, sTAR, products
Steroid biosynthesis in the zona fasciculata and zona reticularis of the adrenal cortex
conversion of cholesterol to pregnenolone is the rate-limiting step in adrenal steroidogenesis & major site of ACTH action in the adrenal
steroidogenic acute regulatory protein (StAR; mitochondrial phosphoprotein) enhances cholesterol transport from the outer to the inner mitochondrial membrane
The major secretory products are DHEA, sulfate, androstenedione, corticosterone, and cortisol
Biosynthesis of Adrenal androgens & cortisol: Zona glomerulosa vs fasciculata & reticularis
zona glomerulosa produces aldosterone & constitutes about 15% of adult cortical volume,
deficient in 17α-hydroxylase activity → cannot produce cortisol or androgens
zonae fasciculata and reticularis are regulated by ACTH; excess or deficiency of this hormone alters their structure & function
both zones atrophy when ACTH is deficient
ACTH is in excess, hyperplasia & hypertrophy of these zones occur
Biosynthesis of Adrenal androgens & cortisol: Pregnenolone, 17α, Lyase, Sulfate, Androstenedione
Cholesterol into mitochondria → P450scc/CYP11A1 cleaves 6-C side chain → pregnenolone
17α-Hydroxylase activity: Converts Pregnenolone to 17-OH-Pregnenolone.
17,20-Lyase activity: Cleaves the side-chain of 17-OH-Pregnenolone to produce Dehydroepiandrosterone (DHEA). DHEA is a weak androgen and a major precursor.
Sulfation: DHEA converted to DHEA-Sulfate (DHEA-S) by the enzyme SULT2A1. DHEA-S is the most abundant steroid in the blood, a stable reservoir.
Androstenedione: DHEA is converted to Androstenedione by the enzyme 3β-HSD (in the adrenal). Androstenedione is a direct precursor to testosterone and estrone
Biosynthesis of Cortisol (4)
Pregnenolone → (via CYP17A1) → 17-OH-Pregnenolone.
17-OH-Pregnenolone → (via 3β-HSD) → 17-OH-Progesterone.
21-Hydroxylation: 17-OH-Progesterone → (via CYP21A2) → 11-Deoxycortisol.
11β-Hydroxylation (Final Step): 11-Deoxycortisol → (via CYP11B1) → Cortisol.
Circadian rhythm related to ACTH & Cortisol secretion
peak in early morning, nadir b/w 12a-4a & PM cortisol interpreted in context of time of day
rhythm changed by: physical stresses (major illness, surgery, trauma, or starvation), psychologic stress (severe anxiety, endogenous depression, the manic phase of manic-depressive psychosis), CNS & pituitary disorders (Cushing syndrome), liver disease, and other conditions that affect cortisol metabolism (chronic renal failure & alcoholism)
RR: Cortisol & ACTH (8a, 4p, & during sleep)
C at 8a: 10-20 μg/mL
C at 4p: 3-10 μg/mL
C during sleep: <5 μg/mL
ACTH at 8a: 10-50 pg/mL
ACTH at 4p: < 20 pg/mL
ACTH during sleep: 5-10 pg/mL
Adrenocorticotropic hormone (ACTH): regulated, administration, chronic stimulation & deficiency
regulated by the hypothalamus & CNS via neurotransmitters, corticotropin-releasing hormone (CRH) & arginine vasopressin (ADH)
administration → rapid synthesis & secretion of steroids (plasma levels of these hormones rise within minutes)
Chronic ACTH stimulation → adrenocortical hyperplasia & hypertrophy;
ACTH deficiency → decreased steroidogenesis & accompanied by adrenocortical atrophy, decreased gland weight, and decreased protein and nucleic acid content
Adrenocorticotropic hormone (ACTH): Stresses, cortisol secretion, 3 mechanisms
Plasma ACTH & cortisol are secreted within minutes following the onset of stresses → abolish circadian periodicity if the stress is prolonged
Cortisol secretion is closely regulated by ACTH, & plasma cortisol levels parallel those of ACTH
3 mechanisms of neuroendocrine control:
Episodic secretion & the circadian rhythm of ACTH
Stress responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis
Feedback inhibition by cortisol of ACTH secretion
Adrenocorticotropic hormone (ACTH): IL-1 & Cortisol
Interleukin-1 (IL-1) stimulates ACTH secretion
Cortisol inhibits IL-1 synthesis
Cortisol transport: Percentages, free cortisol, CBG, antibody, salivary cortisol
10% of the circulating cortisol is free, 75% is bound to CBG, and the remainder is bound to albumin
The plasma free cortisol level: 1 μg/dL; this bio active cortisol is regulated by ACTH
Corticosteroid-binding globulin (CBG) is produced by the liver, and binds cortisol with high affinity
CBG in plasma cortisol-binding capacity: 25 μg/dL.
[Total plasma cortisol] rise above this level → free concentration rapidly increases & exceeds usual fraction of 10% of the total cortisol
There isn’t a “good” antibody to measure free cortisol levels
Salivary cortisol: an estimate of the free cortisol level
Cortisol transport: Factors affecting CBG levels
CBG levels are increased in high-estrogen states (pregnancy; estrogen or oral contraceptive use), hyperthyroidism, diabetes, certain hematologic disorders, and on a genetic basis.
CBG concentrations are decreased in familial CBG deficiency, hypothyroidism, and protein deficiency states such as severe liver disease or nephrotic syndrome.
Cortisol activity: Glucocorticoid receptor & Liver (3)
GR: glucocorticoid (nuclear steroid) receptor, modulates gene expression in target cells
Liver:
Increased expression of gluconeogenic enzymes, phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, & fructose-2,6-bisphosphatase
Maintains plasma glucose during fasting (anti-hypoglycemic action)
Increases plasma glucose during stress (hyperglycemic action)
Cortisol activity: Adipose Cells, Skeletal muscle, Pancreas, Skin
adipose: permissive for lipolytic signals (GH) → elevated plasma FFA to fuel gluconeogenesis
skeletal muscle: degrade fibrillar muscle → amino acid substrates for gluconeogenesis
pancreas: inhibit insulin secretion
skin: Glucocorticoids in excess inhibit fibroblasts → loss of collagen & connective tissue → thinning of the skin, easy bruising, stria formation, & poor wound healing
Cortisol activity: Immune system (3) & Mood (3)
immune: Inhibits monocyte proliferation and antigen presentation; decreased production of IL-1, IL-6, and TNFα
Demargination of neutrophils by suppressing the expression of adhesion molecules
Inhibition of inflammation by inhibiting PLA2, → inhibiting production of leukotrienes & prostaglandins; suppresses COX-2 expression
Mood: Eucortisolemia maintains emotional balance, Increases appetite, Suppression of REM sleep
Cortisol activity: Thyroid & Bone (4)
thyroid function: TSH synthesis & release are inhibited by glucocorticoids, & TSH responsiveness to TRH is frequently subnormal
Bone:
Glucocorticoids directly inhibit bone formation → decreasing osteoblast proliferation & the synthesis of RNA, protein, collagen, and hyaluronate
→ markedly reduce intestinal calcium absorption → lower serum calcium → promotes a state of secondary hyperparathyroidism to maintain the serum calcium within the normal range
supraphysiologic doses of glucocorticoids stimulate bone resorption (via activation of the receptor activator of nuclear factor kappa B (RANK)-ligand/RANK signaling that is osteoclastogenic) → osteolysis & increased biochemical markers of bone turnover
Glucocorticoid-induced osteoporosis
Adrenal androgens: Precursors & Conversions
Androstenedione, DHEA, & DHEA sulfate: precursors for peripheral conversion to the active androgenic hormones, testosterone (T) & dihydrotestosterone (DHT)
DHEA sulfate secreted from the adrenal → DHEA → in peripheral tissues to androstenedione (immediate precursor of the active androgens)
Adrenal androgens: Effects in Males (2) vs Females (4)
Males:
adrenal androstenedione → T (< 5% of the production rate of this hormone) → physiologic effect is negligible
adult males: excessive adrenal androgen secretion has no clinical consequences; boys: premature penile enlargement & early development of secondary sexual characteristics (Hercules)
Females
adrenal substantially: total androgen production by the peripheral conversion of androstenedione to T
follicular phase of the menstrual cycle: adrenal precursors account for 2/3 of T production and 1/2 of DHT production
During midcycle, the ovarian contribution increases, & the adrenal precursors account for only 40% of T production
abnormal adrenal function: Cushing syndrome, adrenal carcinoma, and congenital adrenal hyperplasia → excessive secretion of adrenal androgens, & their peripheral conversion to T results in androgen excess
ACTH & Cortisol Expected values: Plasma ACTH (1), Plasma Cortisol (6)
ACTH: diagnosis of pituitary-adrenal dysfunction: IRMA or ICMA is 9-52 pg/mL
Cortisol:
With radioimmunoassay 8a: 3-20 μg/dL & average 10-12 μg/dL.
4p: half of morning values
10p-2a: < 3 μg/dL
Stress: increases in patients who are acutely ill, during surgery, & following trauma (> 40-60 μg/dL)
[Total plasma cortisol] elevated w increased CBG-binding capacity (commonly when circulating estrogen levels are high eg, during pregnancy): 2-3x higher than normal
[Total plasma cortisol] increased in severe anxiety, endogenous depression, starvation, anorexia nervosa, alcoholism, & chronic kidney disease
Cortisol deficiency: Addison’s, etiology, affects, clinical presentations, test
Addison’s disease = adrenal insufficiency
Autoimmune etiology: involved in polyendocrine autoimmune syndromes (with pancreas and thyroid)
Affects all adrenal steroids including mineralocorticoids (aldosterone) & androgens
Hypoglycemia, hyperkalemia, hyponatremia. nausea, hyperpigmentation
Cosyntropin stimulation test useful
Cosyntropin is hR ACTH fragment with biological activity toward adrenal receptor
Low dose stimulation (1μg subQ injection) → cortisol peak of > 18 μg/dL in 1-2 hrs
Primary Adrenal insufficiency (Addison’s disease)
Deficient adrenal production of glucocorticoids or mineralocorticoids → adrenocortical insufficiency
consequence of destruction or dysfunction of the cortex (primary adrenocortical insufficiency/Addison disease) or secondary to deficient pituitary ACTH secretion (secondary adrenocortical insufficiency)
Glucocorticoid therapy is the most common cause of secondary adrenocortical insufficiency
Primary Adrenal insufficiency: Cortisol & Mineralocorticoid deficiency, Chronic primary adrenal sufficiency
Cortisol deficiency causes weakness, fatigue, anorexia, nausea and vomiting, hypotension, hyponatremia, and hypoglycemia.
Mineralocorticoid deficiency produces renal sodium wasting & potassium retention → severe dehydration, hypotension, hyponatremia, hyperkalemia, & acidosis.
Chronic primary adrenocortical insufficiency: symptoms are hyperpigmentation, weakness & fatigue, weight loss, anorexia, & GI disturbances
Primary adrenal insufficiency: Acute adrenal crisis, Secondary adrenocortical insufficiency
Acute adrenal crisis: state of acute adrenocortical insufficiency & occurs in patients with Addison's disease who are exposed to the stress of infection, trauma, surgery, or dehydration due to salt deprivation, vomiting, or diarrhea
Secondary adrenocortical insufficiency: ACTH deficiency result of exogenous glucocorticoid therapy
Pituitary or hypothalamic tumors, and their treatment, are the most common causes of naturally occurring pituitary ACTH hyposecretion
Congenital Adrenal Hyperplasia (CAH): Deficiency, virilization, severe forms, detection
P450c21, 21α-hydroxylase deficiency accounts for 95% of CAH
Virilization of fetus due to androgen excess
Exacerbated by high ACTH (loss of negative feedback)
Clitoromegaly
Females may require surgery to correct fused labia; Males: “Hercules”
Severe forms are salt-wasting (why?)
Detected by neonatal testing of (elevated) 17-beta-hydroxyprogesterone levels
Rare forms of CAH (4)
11-Beta hydroxylase deficiency: no symptoms associated with adrenal crisis, but are subject to others (HTN) due to salt retention and ambiguous genitalia in females.
17a-hydroxylase deficiency: ambiguous external genitalia in males & lack of pubertal development or menstrual cycles (amenorrhea) in females.
3-Beta-hydroxysteroid dehydrogenase deficiency: ambiguous genitalia in males and females. In both genders it can lead to salt-wasting.
StAR (Steroidogenic Acute Regulatory Protein) Congenital lipoid adrenal hyperplasia: may cause early death due to adrenal crisis
Males have ambiguous genitalia. Both males and females, if they survive, would likely be infertile.
Cortisol excess: Cushing syndrome vs disease, ectopic ATCH, signs, detection
Cushing’s syndrome = cortisol excess from any cause
Cushing’s disease = cortisol excess from pituitary adenoma
Ectopic ACTH syndrome: paraneoplastic syndrome
Classical signs: striae, supraclavicular & other fat deposits (buffalo hump, moon-face)
Delayed wound healing & impaired immune response
Plasma ACTH, salivary and 24 h urinary cortisol measurements are useful to detect excessive secretion
Dexamethasone suppression test
test may be informative for Cushing’s syndrome
Dexamethasone has potent negative feedback on ACTH release
Low dose: 8a cortisol value in normal is < 2 μg/dL, AM cortisol > 14 = suspected Cushing’s syndrome
High dose: 8 mg, most people with Cushing’s disease suppress, those with ectopic ACTH syndrome do not
Cushing’s syndrome & disease: Excess, therapy, spontaneous, classification
Chronic glucocorticoid excess → constellation of symptoms & physical features known as Cushing syndrome
It is most commonly iatrogenic, resulting from chronic glucocorticoid therapy
Spontaneous Cushing syndrome: abnormalities of the pituitary or adrenal gland or a consequence of ACTH or CRH secretion by nonpituitary tumors (ectopic ACTH syndrome; ectopic CRH syndrome)
Cushing disease is defined as the specific type of Cushing syndrome due to excessive pituitary ACTH secretion from a pituitary tumor
Classified as ACTH-dependent/ACTH-independent
Expected diurnal variation in Cortisol (1), ACTH (3), Testosterone (1)
Cortisol: highest level in early morning → steep decline → gradual decline through evening
Cushing’s syndrome: high levels in the evening (late-night salivary cortisol)
ACTH: just like cortisol but with sharper spikes
Addison's: High ACTH due to lack of cortisol feedback
Secondary Adrenal Insufficiency (Pituitary failure): Low or "inappropriately normal" ACTH.
Cushing's Differential: An elevated or high-normal ACTH at 8a suggests ACTH-dependent Cushing's (pituitary or ectopic tumor); suppressed ACTH suggests ACTH-independent (adrenal tumor)
testosterone: just like cortisol but ~20-35% more in morning
diurnal variation diminishes with age: flatter curve
Adrenal medullary hormones: Catecholamines, norepinephrine, pheochromocytoma
Catecholamines: Neuroendocrine hormones (Epinephrine & Norepinephrine)
Metabolites are Norepinephrine and Normetanephrine (Metanephrines)
Pheochromocytoma:
Neuroendocrine tumor overproduces catecholamines & causes severe HTN
Adrenals are difficultly imaged
Plasma & urine metanephrine measurements are sensitive & specific for pheochromocytoma
Adrenal ‘incidentalomas’ are commonly found, incidentally
Ovarian development & function: 6 weeks, secretion, LH & FSH, puberty
At 6 weeks gestation, fetus is sexually bi-potential
Estrogen secretion begins in fetal ovary
Fetal LH & FSH peak midterm & fall to low levels at birth
Puberty: hypothalamic GnRH stimulates increases in LH & FSH (including nocturnal rise in LH)
Ovary feedback loops: FSH/LH, low/high estradiol, progesterone
FSH/LH release from anterior pituitary induced by GnRH
Follicle-stimulating hormone (FSH): Stimulates maturation & growth of GF
Luteinizing hormone (LH): Stimulates ovulation & Induces progesterone secretion (peak) by ovary
Low [estradiol] during menstruation & mid-follicular phase: Provides (-) feedback to GnRH, FSH & LH secretion
High [estradiol] midcycle: Provides (+) feed forward for FSH & LH secretion
Progesterone: Peak in mid-luteal phase provides (-) feedback to GnRH,
FSH & LH secretion
![<ul><li><p><span style="color: rgb(0, 0, 0);"><span>FSH/LH release from anterior pituitary induced by GnRH</span></span></p></li><li><p><span style="color: rgb(0, 0, 0);"><span>Follicle-stimulating hormone (FSH): Stimulates maturation & growth of GF</span></span></p></li><li><p><span style="color: rgb(0, 0, 0);"><span>Luteinizing hormone (LH): Stimulates ovulation & Induces progesterone secretion (peak) by ovary</span></span></p></li><li><p><span style="color: rgb(0, 0, 0);"><span>Low [estradiol] during menstruation & mid-follicular phase: Provides (-) feedback to GnRH, FSH & LH secretion</span></span></p></li><li><p><span style="color: rgb(0, 0, 0);"><span>High [estradiol] midcycle: Provides (+) feed forward for FSH & LH secretion</span></span></p></li><li><p><span style="color: rgb(0, 0, 0);"><span>Progesterone: Peak in mid-luteal phase provides (-) feedback to GnRH,</span></span><span style="color: rgb(0, 0, 0);"><br></span><span style="color: rgb(0, 0, 0);"><span>FSH & LH secretion</span></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/a1c4c7a4-daa2-42b2-a964-facac1c245ae.png)
Estrogens: Estradiol (3), types of receptors (3)
Estradiol has many beneficial effects on:
Vascular endothelium (& heart): lower rate of CVD in women until menopause
Maintenance of bone & bone mineral density (BMD): Single most powerful determinant of BMD in men & women
Muscle & CNS
At least 2-3 types of estrogen receptors are expressed in men and women
Estrogen receptor alpha (ESR-1): nuclear steroid receptor transactivating proliferation signals
Estrogen receptor beta (ESR-2): nuclear steroid receptor transactivating differentiation signals and may be activated by an androgen metabolite as its primary ligand
GPR30? a G-protein coupled receptor – Discovered here in 2007 Go Lobos! Yet highly controversial
Ovarian hormones: Androgens, Activins, Relaxin
Androgens: androstenedione, dehydroandrostenedione, testosterone & dihydrotestosterone (DHT)
Important for female libido
Excess secretion causes hirsutism & extreme cases virilization
Activins: induce FSH secretion & steroidogenesis
Relaxin: prepares for delivery by loosening the muscles & ligaments in pelvis
Ovarian hormones: Inhibins A & B (folliculostatin; function, FSH, A vs B, secretion)
inhibits FSH
FSH stimulates the secretion of inhibins from the granulosa cells of the ovarian follicles in the ovaries
Inhibin B reaches a peak in the early- to mid-follicular phase, & 2nd peak at ovulation
Inhibin A reaches its peak in the mid-luteal phase
Inhibin secretion is diminished by GnRH & enhanced by insulin-like growth factor-1
Androgens: Male sex differentiation, andropause
Weeks 6-7: Bipotential fetus develops testes due to SRY gene on Y chromosome.
Week 10: Leydig cells produce androgens (testosterone) under hCG influence.
Prenatal: Sertoli cells secrete Müllerian Inhibiting Hormone (MIH), causing regression of female reproductive structures.
Andropause: Gradual, age-related decline in testosterone secretion in some men.
Androgens: LH, FSH, inhibin, GnRH
LH → Stimulates Leydig cells → Testosterone production (≈95% of androgens).
FSH + Testosterone → Activate Sertoli cells → Support spermatogenesis.
Sertoli cells also produce inhibin, which with testosterone provides negative feedback on pituitary/hypothalamus (GnRH).
GnRH is released in pulsatile manner with a nocturnal surge.
Androgens: Transport, diurnal rhythm, regulation, FSH action, Androgen receptor
Transport: Testosterone is 95% protein-bound (45% to SHBG, 50% to albumin); only 5% is free and active.
Diurnal Rhythm: Highest in early morning, lowest around midnight.
Regulation: Testosterone and estradiol provide negative feedback to inhibit Leydig cell production (via LH suppression).
FSH Action: Acts on Sertoli cells to boost protein synthesis, inhibin secretion, and androgen receptor (AR) expression.
Androgen Receptor Effect: Activated ARs trigger synthesis of FSH receptors on Sertoli cells, enhancing FSH sensitivity.
Androgens: Inhibin, intracellular metabolism, risks of excess T, anabolic, lupron
Inhibin Function: Secreted by Sertoli cells to provide specific negative feedback on pituitary FSH secretion.
Intracellular Metabolism: Testosterone is converted to more potent DHT or to estradiol (E2) in tissues like fat.
Risks of Excess (T/DHT): Polycythemia (↑ HCT), hyperviscosity, prostate enlargement, and aggressive behavior.
Anabolic Steroid Side Effect: Suppresses the HPT axis → testicular atrophy, impotence, and mood/behavior disturbances.
Lupron: A GnRH agonist used to suppress sex hormone production; treats prostate cancer, endometriosis, precocious puberty, and is used for chemical castration.
Sex steroids Endocrinopathies: Hypogonadism, PCOS
Hypogonadotropic hypogonadism: deficiencies of FSH and LH
Hypergonatropic hypogonadism: ovarian failure (elevated FSH ± LH) example: Turner’s syndrome (XO)
Polycystic Ovarian Syndrome (PCOS): characterized by infertility, anovulation, hyperglycemia, hyperlipidemia, hirsutism, treated with metformin
Growth hormone: Somatotropin (AP hormones, size, insulin, T&E, feedback loop)
Anterior pituitary hormones necessary for appropriate growth: TSH,
ACTH, FSH, LH, GHSomatotrophs > 1/3 mass of pituitary: synthesize & release GH
Pancreatic hormone necessary for appropriate growth: Insulin
Steroid hormones necessary for appropriate growth: T&E
Feedback loop involves hypothalamic GHRH (+ regulator), Ghrelin (+
regulator), Somatostatin (- regulator) & other factors
GH: Bio activity, surrogate marker, assay
Biological activity:
Insulin antagonist: promotes gluconeogenesis & lipolysis
Anabolic effect on muscle increasing Pi & N uptake
Induces Insulin-Like Growth Factor-1 (IGF-1, old name
Somatomedin C) synthesized by the liver
IGF-1 is a good surrogate marker
IGF-1 assay is preferred method assessing deficiency or excess GH secretion & marker of overall protein nutritional status
GH: test, deficiency, in excess
Preferred test for autonomous GH secretion by pituitary adenoma:
75g oral glucose load suppression – adenomas are not suppressedGH deficiency: stimulation tests using insulin-induced hypoglycemia
(older) or GHRH + L-arginine/L-arginine + L-DOPAAcromegaly/Gigantism in excess
Prolactin: Uniqueness, stimulate, increases seen, excess
Unique pituitary stress hormone
Does not target other primary endocrine organs
Secretion is under tonic inhibition by dopamine (neurotransmitter/neuroendocrine hormone)
Promotes lactation and suppresses ovulation (via suppression of FSH & LH secretion and action)
TRH, estradiol stimulate prolactin secretion
Increases seen in pituitary adenoma (Prolactinoma), renal failure, PCOS, after exercise
Excess causes hypogonadism, gynecomastia & infertility, and galactorrhea (rarely in men)
RR Prolactin & Testosterone
Prolactin in M: 4-15 ng/mL
Prolactin in F: 5-23 ng/mL
Prolactin > 150 usually = prolactinoma
Testosterone M adult: 240-950 ng/dL
Testosterone F adult: 8-60 ng/dL
Pituitary ademonas
Functioning PAs: secrete hormones: most common are Prolactin secreting, followed by GH and TSH
Non-functioning PAs: do not secrete hormones
Surgery is often needed to preserve eyesight (PAs press on optic chiasma causing double-vision)
Surgical removal of pituitary masses can cause DI followed by SIADH (biphasic) & DI followed by SIADH, followed in turn by DI (triphasic)
Copeptin: secreted along with ADH & longer circulating half-life than ADH
Is the “C-peptide” of ADH
Chromatography: Forms, TLC, HPLC, GC/GCMS
All forms feature a stationary and mobile phase, but bio samples with drugs or other lipophilic compounds must 1st be extracted into an organic solvent
Thin layer chromatography (TLC): stationary phase is usually silica gel or aluminum oxide attached to inert support; mobile phase is an organic solvent
Liquid chromatography (HPLC): silica or hydroxyapatite media and polymer resins: stationary phase is a column packed with beads coated with silica or hydroxyapatite media and polymeric resins such as polystyrene divinylbenzene
Gas chromatography GC/GCMS: Stationary phase is a long capillary column coil packed with immobilized porous polymers or alumina, mobile phase is a gas (H2) carrying vaporized compounds of interest
Chromatography: Principle, compound separation, standards, retention time (Rf)
Principle of chromatography: compounds can be separated with high resolution according to whether they have high, low or intermediate affinities for either the stationary or mobile phase (or both)
Compounds are separated into spots (TLC) or rings (liquid/gas) as they are pumped or carried through the stationary phase that can be detected visually (thin-layer) or by other detection techniques (N/P, flame ionization, mass spectroscopy)
Rf (how long is compound characteristically retained on stationary phase in minutes) & help ID unknown compounds
Standards are run for comparison of retention times & help calculate [unknown]
Thin-layer chromatography (5)
Lanes 1, 2, 3, and 4 are preloaded standards, extracted urine sample disc fits into the black circles
Toxi-Lab A and B TLC “plates” are placed in jar where a small amount of solvent is wicked up to their tops
Plates are developed in various solution stages (I-IV) and subjected to UV radiation
Match to standard (¡patrones!) at each stage enables qualitative ID
Relative migration factor Rf: ratio of how far drug/metabolite migrates as compared with solvent at top of plate
Mass spectroscopy: Components, steps, tandem
ion source: molecules will be ionized (charged)
mass analyzer: filter & separate molecules based on mass using electrical currents
detector: detect current that is produced by ionized molecule(s) & transform it into a mass
5 steps: ionization, acceleration, deflection, detection, data analysis/libraries
tandem mass spec: used to reduce changes of getting interfering compounds that would pollute signal of molecule of interest
Biochemical & Physiological changes in Pregnancy (10)
Expansion of plasma volume by 85% of pre-conception values
10% decrease in ECF osmolality at end of term
Dilutional but not absolute decrease in RBC/PCV
Modulation of the Renin-Angiotensin-Aldosterone Axis by Relaxin & hCG resulting in increased ADH secretion, sodium and water retention
Progesterone causes K+ retention
Increased GFR helps balance sodium from increase in aldosterone
Increased demand for and risk of iron/iodine deficiency (as well as protein, calories, calcium, etc.)
2-fold increase in calcium absorption
Kisspeptin from hypothalamus rises 10,000 x in plasma: stimulator of GnRH with antimetastatic activity
Rise in TRH causes increased total thyroid hormone
Human chorionic gonadotropin (hCG): blastocyst, urine test, diagnosis, placenta
Glycoprotein hormone produced by blastocyst that will develop into a placenta
Urine test is more sensitive for detection of early pregnancy as compared with serum
Used to establish “diagnosis” of pregnancy (as early as 6-12 days post-fertilization)
hCG is hyperglycosylated before placenta forms, & not reactive with POC testing kits
hCG: during term, peaks, double time, low, cross reactivity, estrogen
Peaks at 10 weeks gestation, declines by midterm to a lower plateau
doubling time (in serum) in 1st trimester (2-3 days) is informative in ectopic pregnancy
levels remain low in ectopic pregnancy (do not meet doubling time criterion – typically ‘flat-line’)
hCG cross reactivity with TSH receptor
Primary estrogen secreted in pregnancy is estriol
Routine testing during pregnancy: Urinalysis (3)
screen for pre-eclampsia, gestational diabetes, infection
pre-eclampsia triad: HTN, edema, proteinuria
gestational diabetes: test for GDM at 24-26 weeks for pregnant women without prior diabetes
Anencephaly & Spina bifida: Risk factor, screening, age, AFP
risk factor: folate status with genetic factors
all women should be offered a screen @ 15-20 weeks gestation
Knowing precise gestational age is important in interpreting results
AFP is elevated in NTCDs (& hepatocellular cancer), & low in Down syndrome
Anencephaly & Spina bifida: MSAFP (what, concentration, rising, fetal)
Alpha-fetoprotein measured first in maternal serum (MSAFP), in amniotic fluid, & fetal plasma (in MoM – multiples of the median)
MSAFP concentration < in amniotic fluid or fetal plasma
MSAFP rises in early pregnancy, peaks between 28 and 32 weeks of gestation, & then falls
Fetal plasma alpha-fetoprotein peaks between 10-13 weeks of gestation → declines exponentially from 14-32 weeks → falls dramatically near term
Non-invasive Prenatal testing for Trisomies (NIPT)
AKA: cell free DNA screening (cf-DNA) for:
Trisomy 21: Down Syndrome
Trisomy 18: Edwards Syndrome
Trisomy 13: Patau Syndrome
Determines gender of fetus & X/Y chromosomal abnormalities
Ratio of cell-free DNA from fetus & mom compared to determine trisomy
Amniotic fluid: Amniocentesis, volume, determine fetal age
Indications for amniocentesis: fetal distress, fetal maturity
assessment, confirmation of prenatal maternal screening result
using cell free placental DNA in maternal bloodVolume: 800-1200 mL at third trimester
Determination of fetal age: creatinine values before 36 weeks (1.5-2.0 mg/dL), after 36 weeks (> 2.0 mg/dL)
Differentiation of amniotic fluid from maternal urine to r/o premature rupture of membranes (PROM) (4)
Creatinine levels are much lower in amniotic fluid as compared with urine
Fern test
pH (amniotic fluid 7.1 to 7.3, normal vaginal fluid 4.5 to 6.0)
Biomarkers: PAMG-1 (placental alpha-macroglobulin), IGFBP-1/PP12
(insulin-like growth factor binding protein-1/placental protein 12),
AFP(alphafetoprotein) + IGFBP-1
Fetal lung maturity (FLM): Maturity, surfactant, L/S, PG, detection, index
fetal lung matures late during the course of pregnancy
Pulmonary surfactant phospholipid (made by type II pneumocytes) after week 35 of pregnancy & is necessary to keep alveoli from collapsing (atelectasis)
Lecithin-sphingomyelin ratio (L/S ratio): indicator of FLM (> 2.0)
Phosphatidyl glycerol (PG): indicator of FLM. Delayed in maternal DM
Thin-layer chromatography detection
Foam stability index (shake up and watch bubbles) & lamellar bodies (uses
PLT channel from automated cell counters)