Unit 1B - Structure and Properties of Substances
1/53
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
What does the Lewis Theory of Bonding state?
Atoms will gain, lose, or share valence electrons in order to obtain a stable octet and will have an electron configuration that is isoelectronic with the closest noble gas
A stable octet refers to full s and p orbitals (total of 8 in valence)
Metallic bonding
Occurs between (same) metal atoms; because metal atoms have a weak electronegativity and hold on their valence electrons, they become delocalized and a “sea” of valence electrons of many atoms move freely among them. The resulting cations are all attracted at the same time to the “sea” of electrons.
Electronegativity (EN)
The relative ability of an atom to attract shared electrons in a bond; EN increases up a group and to the right of a period
Ionization energy (IE)
The quantity of energy required to pull the outermost electron from a gaseous atom; IE increases up a group and to the right of a period
How are ionic bonds formed?
Complete transfer of electrons, full charges on resulting ions
How are polar covalent bond formed?
Unequal sharing of electrons, partial charges of bonded atoms
How are non-polar covalent bonds formed?
Equal sharing of electrons, no charge in bonded atoms (usually occurs between atoms of the same element)
Why do metals have high melting and boiling points?
A large amount of kinetic energy is required to pull the particles apart; the larger number of electrons in the “sea”, the larger the charge on ions, resulting in a stronger attractive force
What are factors that make some metals have higher melting and boiling points than others?
Has to do with the number of valence electrons, increasing across a period (ions have a larger attractive force when the charges are higher)
Why are metals generally good electrical and heat conductors?
Their valence electrons are able to move freely from one atom to another (and conduct thermal energy for the same reason)
Why are metals generally very malleable and ductile?
Because of the electron-sea model; when stress is applied to the ions, they will slide past each other, deforming the compound
Why do ionic compounds have relatively high melting and boiling points?
Because of the structure of the crystal lattice and bonds; the attraction between oppositely charged ions is very strong, requiring a large amount of energy to overcome
Why do some ionic compounds have higer melting and boiling points than others?
Larger charges create stronger attractive forces between ions, however, the main reason is the size of the atoms; when one is significantly smaller than the other (eg. Na and Cl), they are able to be packed more closely together, creating a stronger attractive force
Why are some ionic compounds soluble in water?
For a compound to be soluble in water, the IMFs between the water molecule and the compound must be stronger than the attractive forces among the ions themselves; the water molecules are able to exert enough force to break the ionic bonds
Why are ionic compounds brittle?
As an ionic compound is subjected to stress, this may cause like charges to become aligned, repelling each other and breaking apart the crystal lattice structure
Why does a solid ionic compound not conduct electricity?
The bonds (IMFs) between ions in a solid ionic compound are much stronger than the electrical force, and will not move
Why does a dissolved ionic compound conduct electricity?
When an ionic compound is dissolved in water, the ions are no longer held together by those same attractive forces, and are able to move freely and conduct electrical current
Exceptions for the octet rule (will form an expanded octet)
Third row and heavier elements
Conditions for covalent bonding
Elements must have high ionization energy and electronegativity, as well as space for new bonds to be formed
What are the limitations of Lewis Calculations?
They don’t work for Be, B, and elements in periods 3 or higher
Coordinate covalent bonding
Bonding in which one atom donates both electrons in a covalent bond; once formed is identical to regular covalent bond
Formal charge
Calculated as the difference between the number of valence electrons that ‘belong’ to an atom when bonded in a molecule and the number of electrons that atom has when unbonded; FC is determined for every particular atom (should be close to 0 if not an ion, should be equal to the charge of ion if it is)
Resonance structures
One possible Lewis Structure for a compound that shows the same relative position of atoms but different positions of lone and bonded pairs of electrons; the actual molecule will be a hybrid of its resonance structure
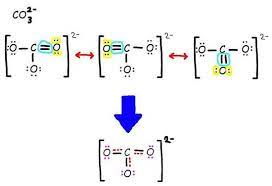
Delocalized Bonding Electrons
Occurs when there is more than one possible position for a double bond in a molecule or ion
Paramagnetic species
Molecules or polyatomic ions can experience a paramagnetic moment because one of their atoms has a lone electron (half-filled orbital)
What determines the properties of molecular substances?
The size and shape of the molecules
What determines molecular shape?
The number of electron domains
How do orbital overlaps have an effect on bonds?
The greater the orbital overlap, the stronger and more stable the bond
Valence Bond Theory
Explains covalent bond formation and molecular shape based on atomic orbital overlap
Who came up with the concept of hybrid orbitals?
Linus Pauling
How much energy do hybrid orbitals have?
Resulting hybrid orbitals are all of the same energy (some intermediate value) of the original combined orbitals
Why do cis and trans isomers exist?
Pi bonds are rigid and restrict rotation of the molecule
Sigma bonds
Result from end-to-end overlap of atomic orbitals
Where does the highest electron density occur?
Along the axis between the two nuclei (sigma bonds), above and below the axis bewteen the two nuclei (pi bonds)
Pi bonds
Bonds that result from the side-to-side overlap of unhybridized p orbitals
Polar molecule
Charge is not distributed evenly between the atoms (dipoles do not cancel out to 0), degree of sharing depends on the difference in electronegativity
Nonpolar molecule
Electrons shared equally between atoms (either a result of nonpolar bonds or symmetry, sum of bond dipoles is 0)
Bond dipole
The difference in electronegativity of atoms, illustrated through arrows
What is the unit of molecular dipoles?
Debye units
Molecules with only C and H atoms are…
Nonpolar
Intermolecular forces
Occur between molecules (weaker than intra. forces)
Intramolecular forces
Occur within molecules (stronger than inter. forces)
What is determined by intermolecular forces?
The bulk physical properties of matter
IMFs are only relevant for…
Substances in condensed phases (liquids, solids)
London Dispersion Forces
The weakest intermolecular force, occurs between all molecules; temporary attractive force that exist between adjacent molecules because of a spontaneous and temporary dipole
Dipole-Dipole Forces
Stronger than LDFs, only polar molecules will experience these with other polar molecules; results from the forces of attraction between two oppositely charged dipoles
Hydrogen “Bonding”
Occur between highly polarized molecules containing N, O, or F atoms; results from the strong attractive force between the H atoms of one molecule and a LP in another
Ion-Dipole Interactions
Attractive forces between an ion and a polar molecule
Viscosity
The ability of a liquid to resist flow (strong IMFs=high viscosity)
Factors determining viscosity
Strength of IMFs, size and shape of a molecule, temperature
Surface tension
The resistance of a liquid to spread out and increase its surface area (strong IMFs=strong surface tension)
Cohesion
The intermolecular attraction between like molecules (strong IMFs=strong cohesion and adhesion)
Adhesion
An attraction between unlike molecules (strong IMFs=strong cohesion and adhesion)
Vapour pressure
The pressure exerted by gaseous molecules above a liquid (strong IMFs=low vapour pressure)