chemical properties of freshwater
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
increased CO2 in the atm is acidifying freshwaters
freshwaters are variable but it’s less obvious compared to oceans - less so
the composition of dissolved substances in freshwater is mostly driven by some of the least soluble substances
true - NaCl in rocks being weathered - NaCl is very soluble so it comes out of the rocks easily
water has a dipole moment which leads to what other properties of H2O?
hydrogen bonding, high specific heat, and high latent heat, surface tension
where do freshwaters matter
in the global carbon cycle - wasn’t considered important for a while though
behavior of freshwater systems
is totally coupled to the physical-chemical properties of water - in terms of atm and vegetation, bog or peatland have different compositions than groundwater wetland in southwestern Mn
what determined freshwater composition
determined by where water comes from: atm vs soils and rocks
ions that are not very soluble
dominate the chemistry of freshwater
freshwater on the global carbon cycle image
almost ½ Pg of dissolved C and ½ Pg of inorganic C delivered to the sea
asymmetric covalent bonds
give water a dipole moment - have partial positive on H and partial negative on O
dipole moments lead to
H-bonding and many unique properties of water
lots of energy required or given off in phase transitions (high latent heat)
water can hold a large amount of heat (high specific heat)
unique aspects of surface tension and density
ice crystals
less dense than liquid water so ice floats
Density characteristics of water
water is 800x more dense than air and more viscous and pure water reaches its max density at 3.98 C
where is the warm water in a lake when it starts to thaw
at the bottom of the lake - density of water changes with temperature - warm water is at surface and becomes less dense and colder water is more dense
water temp in a lake outside of winter
temperature decreases the further down you go - thermal stratification
epilimnion
warm isothermal mixed layer
metalimnion
region where temperature changes rapidly (thermocline) - usually between freezing and liquid and occurs in the middle of the lake depth wise
hypolimnion
deep, cool, high-density water mass
dimitic lake
mixes twice a year in fall and spring
annual temperature changes in dimictic lakes (Lawrence Lake in Michigan)
in Jan., the lake is under ice and the temperature is close to zero - gets warms the deeper you go under ice
thermocline begins to rise up in April and mid to late may is mixed
temp is uniform from top to bottom when lake is mixing
the contours go from top all the way to bottom so it’s mixing and doing gas exchanges with the atm and nutrient exchanges with sediments
Polymictic lake
Clear Lake, CA but similar to Mn lakes - not very deep (less than 4 or 5 m) can mix any time during the year with wind
monomictic lakes
UK Lakes - lakes that don’t form ice so mixes only once - usually between May and September to about 20 m
importance of mixing in lakes
enables gas exchange with atm (methane, CO2 can be release and CO2 can be absorbed)
brings up nutrients from below and promotes production (biomass falls to bottom where it produces nutrients and mixing brings the nutrients back up)
the depth of mixing delineates habitats for organisms/processes in freshwater
Lotic freshwater
rivers, streams, etc. - dynamic physics and chemistry
Lentic freshwater
Lakes, ponds - more stable
wetlands
bogs, fens, peatlands, swamps, etc. - shallow water, lots of plants
littoral zone
the shallow, near-shore area of a lake or pond where sunlight can reach the bottom, allowing aquatic plants (macrophytes) to grow
photic zone
the uppermost layer of a body of water (like an ocean or lake) where sunlight penetrates, allowing for photosynthesis.
Pelagic zone
encompasses the entire open water area of the ocean, excluding the seafloor and coastal areas. It's the largest habitat on Earth, divided into different zones based on depth and sunlight penetration
aphotic zone
the region where there is little or no sunlight
profundal zone
the deepest area in a lake or pond, below the limnetic zone, where sunlight no longer penetrates. - general territory
do organisms sink?
yes - inevitable - they make structures that are denser than water and are negatively buoyant
what will sink in water
density of pure water at 4 degrees C is 1000, so anything greater will sink
does algae sink
algae make fats and oils but they make things that prevent them from fully being at the surface because they can sunburn
sinking and life at low Re
stokes equation - little sings sink slowly, big things sink faster
solvent properties of water
because water has a dual charge, it is chemically attracted to ionic crystals like salt and will bring them into solution
solubility of gases in water
solubility increases as temperature decreases - less molecular activity at low temp decreases the likelihood that gases will escape to the atm
gases dissolve in water better the colder the temps
pressure effect on solubility
solubility increases as pressure increases - think the angler fish story that came to the surface to die
pulling a deep sea fish up to the surface fast explodes its bladder and kills it
annual oxygen dynamics - Lake Minnetonka July through August
oxygen stays at top of lake and makes its way to the bottom likely during a storm only once - no oxygen on the bottom so fish wouldn’t survive here
CO2 sources
atmosphere, soils, rocks and respiration
concentration in atm: 410 ppm and rising
CO2 in water
atmospheric CO2 diffusion into water in described by Henry’s law (c=Kp)
c = concentration
K = constant
p = partial pressure in atm (the pressure pushing it into the liquid)
as the push increases, the concentration increases
is the CO2 concentration in equilibrium with the atmosphere
not necessarily because the CO2 concentration in water is also partially controlled by equilibria with rocks and biological processes such as photosynthesis and respiration
mixing is like shaking a soda can - releases CO2 into the atm
how do CO2 concentrations change throughout the summer
CO2 concentration at water surface is below equilibrium concentration in daytime but then photosynthesis is taking up more than can be replaced - opposite at night - CO2 levels are above equilibrium at night because there is no photosynthesis at bottom but there is respiration
CO2 concentration on surface
below saturation because there is so much photosynthesis
most freshwater with dissolved inorganic carbon
inorganic carbon in freshwater has a pH between 6 and 9 so you have more free carbon (CO2 + H2CO3-)
water is a weak acid/base
a small fraction of water molecules dissociates into protons and hydroxyl groups to form acid and base with equal activities so do not control pH
CO2 is an acid when dissolved in water so it lowers the pH
carbonate precipitation
If CO2 is removed (as in photosynthesis or due to temperature increases), CaCO3 precipitates. If CO2 is added, it will dissolve solid CaCO3. When CO2 is above the equilibrium concentration, it is referred to as “aggressive” CO2.
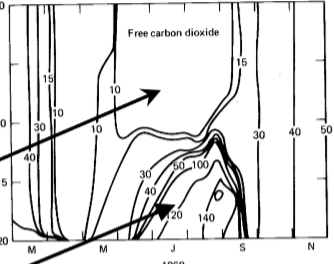
generalized DIC profiles in summer
oligotrophic CO2 controlled by equilibrium with the atmosphere but eutrophic lakes may not be
seasonal dynamics
note drawdown of free CO2 in summer and buildup in the hypolimnion
cations in water and water hardness
water harness is caused by the presence of multivalent ions especially C2+ and Mg2+
hardness is expressed by mg/L of CaCO3 and meq/L
regional water hardness
western and Northeastern US tend to have softer water - midwestern, central, and some southern areas tend to have harder water - hard water is usually linked to limestone or dolomite-rich regions which dissolves Ca2+ and Mg2+
anions - where does alkalinity come from
MeCO3 + CO2 + H2O = Me++ + 2HCO3- where Me refers to “metal
and is usually Ca2+ or Mg2+
other ions contributing alkalinity: borate, phosphate, silicates but these aren’t typically very important because their concentrations are too low
solubility of different ions in water
Ca2+, Mg2+, and CO32- are less soluble than Na+ and Cl-
abundance does not equal solubility - there is more Ca2+ and Mg2+ in rocks and soils, but their salts are less soluble so they precipitate out
Na+ and Cl- are dominant in oceans and often in rivers, while Ca2+ and Mg2+ are more regulated by precipitation reactions and biological cycling
conductivity
electrical conductivity is normalized to 25 degrees C - conductivity of pure water ca. 5 microS - conductivity increases with weatherability
climate and geology
the influence of geology and precipitation on ion dominance - freshwater have less than 0.5% ppt`
rivers and watersheds
shallow soils have short GW residence times while deep soils have long GW residence times
importance of flow paths
deep groundwater has a higher concentration of calcium than saturated soils which has slightly more than unsaturated soils (vadose zone)