b) To recrystallise a sample of benzoic acid
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Theory
• The benzoic acid crystals formed contain impurities
• These impurities are removed by a technique called recrystallisation
Insoluble- removed by filteration
Soluble - removed by recrystallisation
Procedure: step 1
➢ A known mass of the impure benzoic acid crystals are dissolved in the minimum amount of boiling water creating a hot saturated benzoic acid solution
➢ The saturated benzoic acid solution is filtered by gravity filtration using a heated funnel to remove insoluble impurities
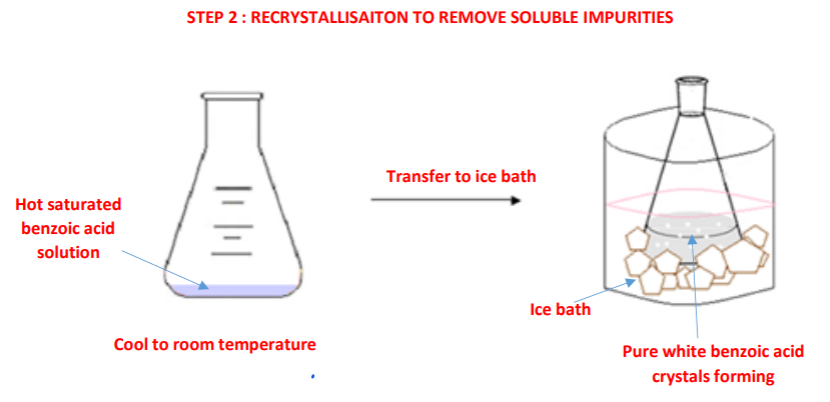
Procedure: step 2
➢ The saturated benzoic acid solution is allowed to cool to room temperature and then placed in an ice bath causing the dissolved benzoic acid crystals to recrystallise out of solution while soluble impurities remain in solution
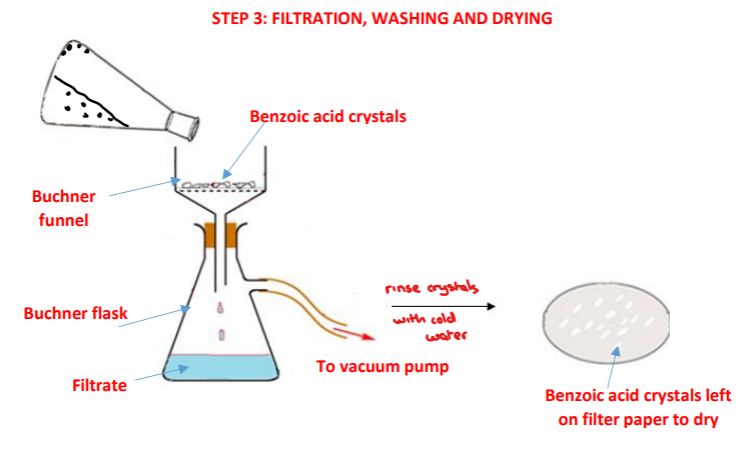
Procedure: step 3
➢ The benzoic acid crystals are filtered using vacuum/suction filtration using filter paper, a Buchner funnel and a Buncher flask
➢ The conical flask is rinsed with the filtrate from the Buchner flask and is re-filtered to ensure maximum yield of benzoic acid crystals are obtained
➢ The benzoic acid crystals are washed with cold water remove any soluble impurities present on the filter paper or on the crystals
➢ The benzoic acid crystals are left to air dry on the filter paper in a warm place or in a desiccator
What impurities could be present in the benzoic acid crystals?
1. Insoluble impurities -that are not dissolved. Example: charcoal
2. Soluble impurities that dissolve. Example: sodium chloride
Why are the benzoic acid crystals dissolved in the minimum amount of boiling water?
• Benzoic acid crystals are very soluble in hot water
• Using the minimum amount of boiling water causes the benzoic acid solution to be saturated
• Cooling this saturated solution will cause maximum amount of pure benzoic acid crystals to recrystallise out of solution
Why is the hot saturated benzoic acid solution filtered?
To remove insoluble impurities
Why is water an ideal solvent for recrystallisation of benzoic acid?
Benzoic acid crystals are very soluble in hot water but only slightly soluble in cold water
Why is the funnel used heated?
To avoid crystallisation of benzoic acid crystals while filtration is taking place
Why is the filter paper fluted?
The surface area of fluted filter paper is greater – the filtration process will be quicker
What precaution should be taken if any benzoic acid crystals form during the filtration?
Using a dropper, add some hot water to dissolve the crystals
In what situation may this filtering stage not be required?
If there are no insoluble impurities present in the sample of benzoic acid
Why is the hot saturated benzoic acid solution cooled?!
To allow recrystallisation to take place i.e. the formation of benzoic acid crystals out of solution
What is recrystallisation?!
Recrystallisation is a process of dissolving crystals in a hot solvent to form a saturated solution and cooling this saturated solution to reform crystals that are more pure – it is based on the principle that solutes are more soluble in hot solvents than in cold ones
Why does this stage remove soluble impurities from the benzoic acid?!
As benzoic acid crystals are only slightly soluble in cold water, they recrystallise out of solution while soluble impurities remain dissolved
Why is the solution first cooled to room temperature before being placed in an ice bath to cool?!
To ensure recrystallisation is complete and obtain the maximum yield of benzoic acid crystals
Note: Larger crystals form when the hot solution is being slowly cooled
What precaution can be taken if crystals are slow to form?!
(1) A “seed” crystal of pure benzoic acid is added to the solution
(2) Scratch the inside of the conical flask with a glass rod
(3) Evaporate some solvent and allow the solution to cool again
What is the purpose of vacuum filtering the solution??
To separate and isolate the benzoic acid crystals away from the solution of soluble impurities
Note: The soluble impurities are more soluble in water than benzoic acid so will not crystallise with the benzoic acid and will pass through the filter paper into solution
Why is vacuum filtration performed instead of gravity filtration??
a) To speed up the filtration process
b) To help dry the crystals
How are the benzoic acid crystals further dried??
Allowed to air dry on the filter paper in a warm place
Give two procedures carried out on the pure dry benzoic acid crystals obtained??
(1) The % yield of purified crystals from the impure crystals is found
(2) The melting point of the purified dried crystals is determined and compared to original impure crystals