L10+11:Chemolithotrophy I and II
1/49
Earn XP
Description and Tags
These flashcards cover key concepts and facts from the notes on chemolithotrophy and hydrogen oxidation, focusing on definitions, processes, and significant discoveries.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
What is chemolithotrophy?
It is the use of inorganic compounds as electron donors in metabolic processes.
How are bacteria extremely versatile in their use of substrates?
Electron donor - can be organic or inorganic
Electron acceptor - can be oxygenic or anyoxygenic
Carbone source - multicarbon or CO2
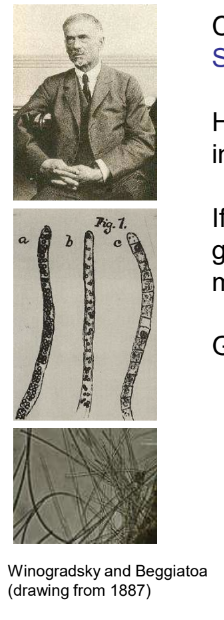
Who introduced the concept of chemolithotrophy and what did they discover?
Sergei Nikolaievich Winogradsky.
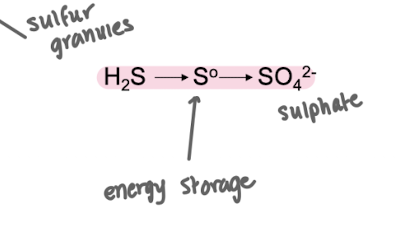
Noticed colourless bacteria with sulfur inclusions in water rich in H2S
If these bacteria are starved for H2S, the sulfur granules disappear and SO42- appears in the medium
Granules reappear when H2S is added
What type of bacterium is Escherichia coli classified as?
Chemoorganotrophic bacterium.
How do chemolithotrophic bacteria obtain energy?
By oxidizing inorganic compounds.
What is nitrification in microbiology?
The oxidation of ammonia to nitrite and then subsequent oxidation of nitries to nitrate, allows growth in the absence of organic C
NH4+ is oxidised by bacteria i.e. Nitrosomonas and then nitrite NO2- is further oxidised to nitrate NO3- i.e. by Nitrobacter.
NH4+ (oxidised) → NO2- (reduced)
NO2- (oxidised) → NO3- (reduced)
The amount of organic matter formed is proportional to the amount of ___________ and dependent on ____-
amount of ammonia, dependent on CO2
What is a Winogradsky column ?
Used to study the interactions of microbes, simulating natural sediment environments , particularly those involved in sulfur and nitrogen cycles,
Enriches microbes by creating natural vertical gradients of oxygen, light, and nutrients that allow different aerobic and anaerobic bacteria to grow in zones suited to their metabolic needs.

What are the main electron donors in Winogradsky columns?
Inorganic compounds such as H2S, S, and NH4+, Mn2+, Fe 2+, CO, H2
Does this reaction have good electron donors and what is the electron acceptor? NH4+ + 1½O2 NO2- + 2H+ + H2O :
Yes, it has a negative free energy, electron acceptor is oxygen: ΔGo’ -275 kJ/reaction
Which reaction is more energy yielding and why?
1: H2 + ½ O2 → H2O
2: Fe2+ + H+ + ¼ O2 → Fe3+ + ½ H2O
The reaction with H2 is more energy yielding because it has a more negative free energy change (ΔGo -237kJ / rxn, spontaneous) compared to the oxidation of Fe2+ (ΔGo -33 kJ / rxn)
yield is equal to the amount of _______ per __________ with the more energy input into a reaction, the more ____________
biomass per mol substrate, more biomass output.
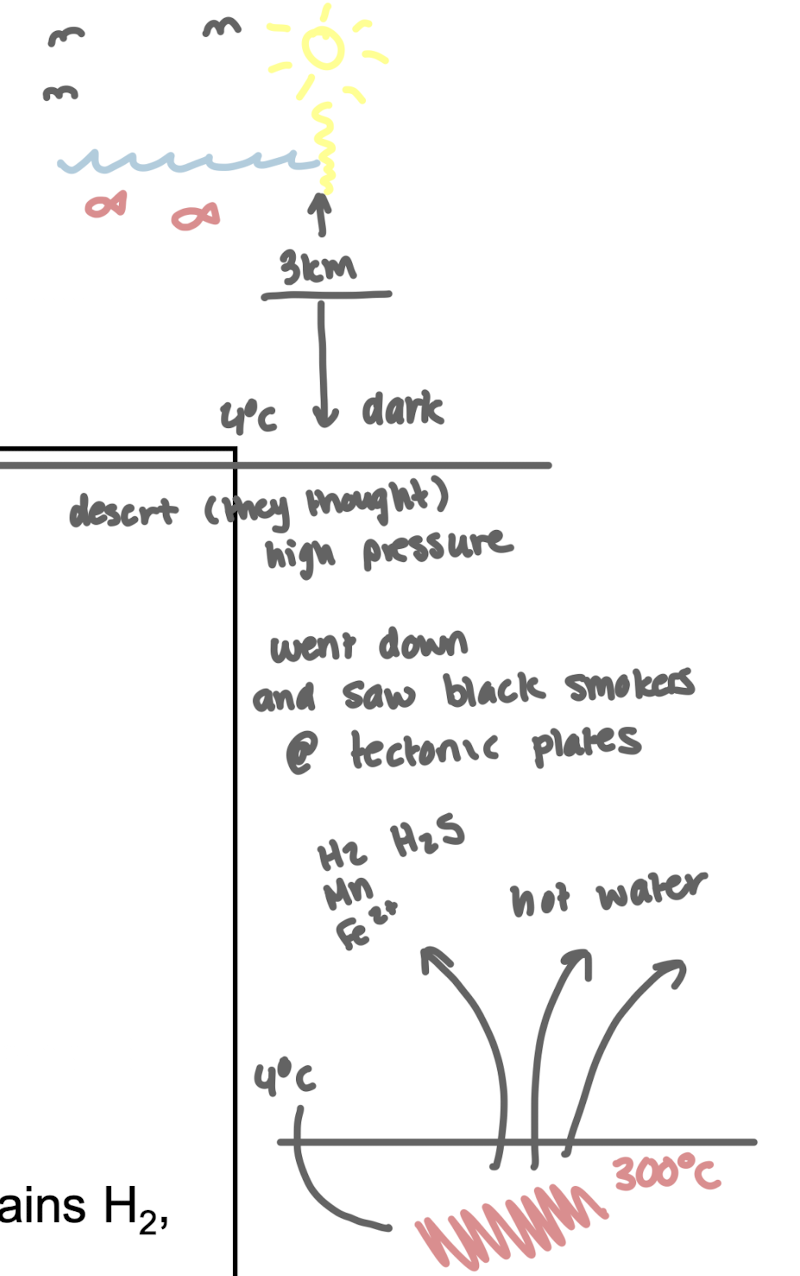
What environmental conditions can support chemolithotrophic life?
Anthropogenic activities, volcanic activity, and hydrothermal vents.
i.e. in hydrothermal vents, black smoker fluid found at tectonic plates (300oC) contains H2, H2S, Mn2+ and CO
Absence of organic matter and no penetration of sunlight supports the survival of chemolithotrophs, which utilize inorganic substances for energy in extreme environments.
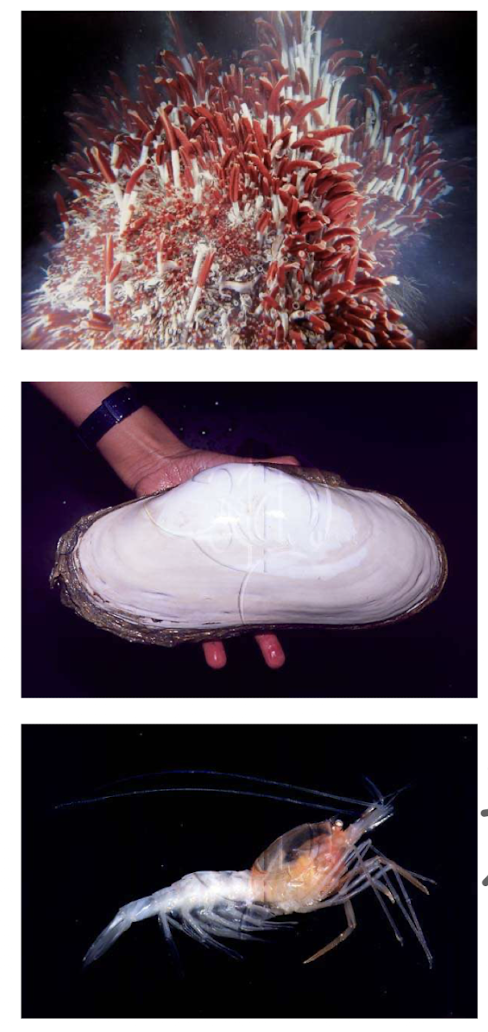
What lifeforms are found around hydrothermal vents?
Despite no sunlight and organic compunds, organisms like chemolithotrophic bacteria and archaea thrive:
Symbiosis b/w chemolithotrophic bacteria and animals present
CO2 fixation via calvin cycle, inorganic substrates serve as ED for ATP generation + reducing power
Why are space agencies like NASA and the ESA interested in the prescense of such chemolithotrophs in these extreme environments? (i.e. no sunlight / lack of OC)
Subsurface liquid water oceans is present on Jupiter’s moons Europa, Ganymede and Callisto as well as Saturn (Enceladus, Dione and Titan, with tidal heating responsible for melting of ice.
Europa’s surface is stained with S compounds, with places on Earth being potential indicators of extraterrestrial life as microbes release So and H2SA
Life is present could resemble the chemolithotroph’s environment around hydrothermal vents.
Chemolithoautotrophic communities drive primary production in dark environments, which may suggest the possibility of extraterrestrial life in subsurface oceans.

What is an example of a chemolithotrophic bacterium and its significance?
Thiobacillus ferrooxidans: Is an Fe2+ oxidising bacteria which grows at low pH (2) and plays a crucial role in bioleaching and metal extraction:
Mining can release high conc. of Fe2+ from pyrite (FeS2) where it readily oxidises to insoluble Fe3+ (rust) at neutral pH in oxygenic cond.
What role does hydrogen play in chemolithotrophic processes?
It is often oxidized to generate ATP and reducing power.
Marine sediments are a major source of _____ which is produced by _________ under _______growth conditions
Sulfide (H2S) , produced by sulfate reducing bacteria, under anaerobic growth conditions.
Issue is H2S is rapidly oxidised under aerobic cond.
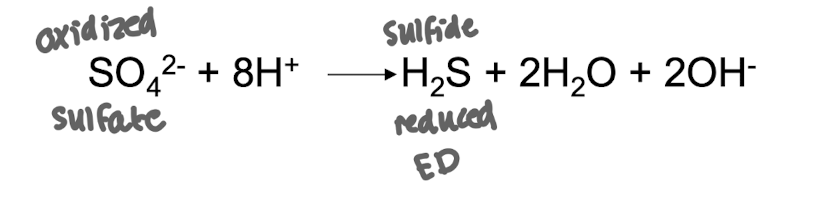
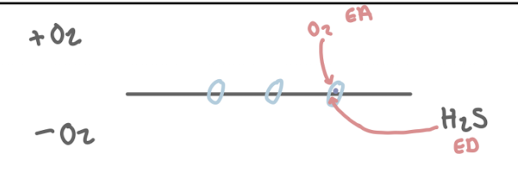
*O2 is the EA to oxidise sulfide
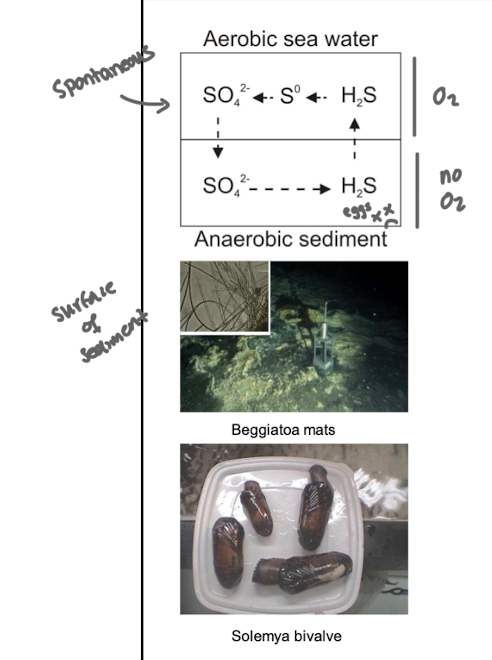
What kind of metabolism do sulfide-oxidizing chemolithotrophs exhibit?
They are found at interfaces between anaerobic sediment and aerobic water.
Often they form dense mats to stabilise the interface and avoid mixing of aerobic and anaerobic layers e.g. Beggiatoa
Symbiosis with bivalves that burrow or move - crossing the anoxic-oxic interface
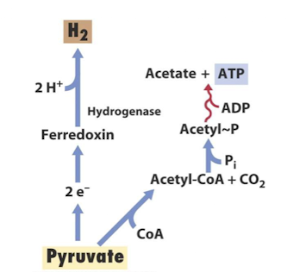
What is a major source of H production?
Anaerobic metabolism:
Fermentation of pyruvate produces acetate and H2 which can be utilized by hydrogen-utilizing microbes for energy.
What is the role of hydrogenase in hydrogen-oxidizing bacteria?
A nickel containing enzyme oxidize hydrogen and supply the cell with reducing power and ATP.
H2 +1/2 O2 → H2O ΔGo -237 kJ / rxn)
What major nutrient cycle do chemolithotrophic reactions contribute to?
The nitrogen cycle, as they are involved in the conversion of nitrogen compounds.
What is the energy yield of hydrogen oxidation compared to ferrous iron oxidation?
Hydrogen oxidation is more energy-yielding than the oxidation of ferrous iron.
What is the Calvin cycle’s role in chemolithotrophy?
It facilitates CO2 fixation to produce organic compounds using ATP and NADH.
What is Xanthobacter flavus and Ralstonia eutropha known for?
They are bacteria capable of oxidizing hydrogen and capable of autotrophic growth, fixing CO2
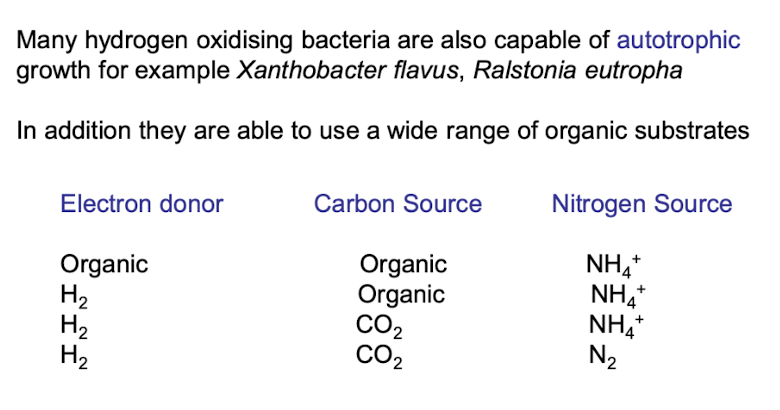
what is mixotrophic growth?
Simulataenous use of inorganic and organic compounds as ED and C source
What is released during mining that leads to acid mine drainage?
Ferrous iron (Fe²⁺) released from pyrite (FeS₂) oxidation, producing sulfuric acid.
This process causes environmental degradation by lowering pH and releasing heavy metals.
Why is sulfide (H₂S) rapidly oxidised in aerobic conditions?
It spontaneously oxidises when exposed to oxygen, making it difficult for anaerobic sulfide producers to maintain sulfide levels.
Where are sulfide-oxidising chemolithotrophs typically found?
At the interface between anaerobic sediment and aerobic water.
What strategies do some sulfide-oxidising bacteria use to survive at oxic–anoxic boundaries?
Forming dense mats (e.g., Beggiatoa) or forming symbioses with burrowing animals like bivalves.
What major biological process generates hydrogen?
Anaerobic fermentation (e.g., pyruvate → acetate + H₂).
Why does hydrogen oxidation typically occur at aerobic/anaerobic interfaces?
Because H₂ is rapidly oxidised under aerobic conditions and must be accessed near where it is generated anaerobically.
What is the main biological source of ammonia (NH₄⁺)?
Decomposition of nitrogen-containing organic compounds such as amino acids and nucleotides.
What electron donors and carbon sources can hydrogen-oxidising bacteria use?
They may use H₂ or organic molecules as donors and either CO₂ or organic carbon as carbon sources.
What enzyme catalyses hydrogen oxidation?
Hydrogenase (nickel-containing).
What two types of hydrogenases do many hydrogen oxidisers possess?
Membrane-bound hydrogenase: generates proton motive force
Cytoplasmic hydrogenase: produces reducing power (NADH)
What metabolic cycle is responsible for CO₂ fixation in hydrogen oxidisers?
The Calvin cycle
Why is CO oxidation not leading to atmospheric accumulation of CO?
Carboxydotrophic bacteria oxidise CO → CO₂, preventing its buildup.
What is the electron acceptor for CO oxidation in carboxydotrophic bacteria?
Oxygen, via CO dehydrogenase feeding electrons into the electron transport chain.
What are the major oxidation states of sulfur used in chemolithotrophy?
H₂S = –2
S⁰ = 0
S₂O₃²⁻ = +2
SO₃²⁻ = +4
SO₄²⁻ = +6
Why is H₂S oxidation highly energy-yielding?
It involves an eight-electron transfer to oxygen, enabling significant proton pumping.
Why do sulfur-oxidising bacteria store elemental sulfur granules?
To use them as energy storage when H₂S becomes limiting.
What environments are rich in reduced inorganic electron donors (H₂, H₂S, CO, Fe²⁺)?
Hydrothermal vents (black smokers)
What type of symbiosis occurs near hydrothermal vents?
Animals (e.g., tubeworms) host chemolithoautotrophic bacteria providing fixed carbon - mutalism
Why are hydrothermal vent ecosystems independent of sunlight?
Primary production is driven by chemolithoautotrophy, not photosynthesis
Why is Fe²⁺ oxidation energetically weak?
The potential difference between Fe²⁺/Fe³⁺ and O₂/H₂O is very small, yielding little energy.
What adaptation allows iron-oxidising bacteria to use Fe²⁺?
Growth in acidic environments (pH ~1–2), where Fe²⁺ remains stable.
What protein catalyses Fe²⁺ oxidation in the periplasm?
Rusticyanin is a copper-containing protein that facilitates electron transfer during the oxidation process.
Why do iron oxidisers rely heavily on reverse electron flow?
To generate NADH for CO₂ fixation, since Fe²⁺ electrons enter the chain at high potential.
What natural advantage do acidophilic iron oxidisers have for ATP synthesis?
A natural pH gradient (external pH 1–2, internal neutral) creates a strong proton motive force by allowing protons to flow back into the cell, driving ATP synthesis.