Ionic bonding
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
What is an ionic bond?
The electrostatic force of attraction between oppositely charged ions
What are ions?
Elements that have gained or lost electrons to obtain a full outer shell
What are anions?
Negative ions formed by elements gaining electrons
All non-metals form negative ions
What are cations?
Positive ions formed by elements losing electrons
All metals form positive ions
What are the charges of ions in group 1?
1+
What are the charges of ions in group 2?
2+
What are the charges of ions in group 3?
3+
What are the charges of ions in group 5?
3-
What are the charges of ions in group 6?
2-
What are the charges of ions in group 7?
1-
Why are elements in group 8/0 unreactive to ionic bonding?
Because they do not need to gain or lose any electrons as they already have a full outer shell
Why do compounds with giant ionic lattices have high melting & boiling points?
Because they have a giant structure with strong electrostatic forces of attraction between oppositely charged ions
This means that a lot of energy is needed to overcome these forces of attraction, therefore giving the compound a high melting & boiling point
Why do ionic compounds not conduct electricity when they are solids?
Because the ions are in fixed positions & therefore are not free to move
Why do ionic compounds conduct electricity when they are molten or in aqueous solution?
Because they have charged particles that are free to move
What is the formula for a hydrogen ion?
H+
What is the formula for a hydroxide ion?
OH-
What is the formula for ammonium?
NH4+
What is the formula for carbonate?
CO32-
What is the formula for nitrate?
NO3-
What is the formula for sulfate?
SO42-
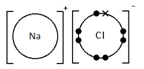
Give a dot & cross diagram to show the formation of the ionic compound Sodium Chloride (NaCl)
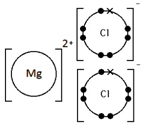
Give a dot & cross diagram to show the formation of the ionic compound Magnesium Chloride (MgCl2)
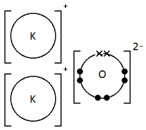
Give a dot & cross diagram to show the formation of the ionic compound Potassium Oxide (K2O)
Give a dot & cross diagram to show the formation of the ionic compound Calcium Oxide (CaO)
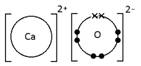
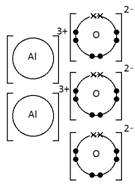
Give a dot & cross diagram to show the formation of the ionic compound Aluminium Oxide (Al2O3)
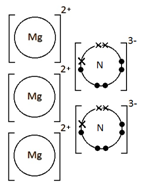
Give a dot & cross diagram to show the formation of the ionic compound Magnesium Nitride (Mg3N2)