Analytical Chemistry 2
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Q: What is countercurrent extraction?
A:
A technique for separating solutes,
based on their differing solubility
in two immiscible liquids,
where one phase continuously moves through the other.
Q: How does countercurrent extraction differ from conventional solvent extraction?
A: Countercurrent extraction is
a continuous process
that allows for multiple partitioning steps,
improving efficiency and reducing solvent use,
While conventional methods typically involve batch processes.
Q: What is the primary objective of countercurrent extraction?
A:
To separate two or more solutes from each other
through a series of partitions between two liquid phases
with different distribution coefficients
Q: What are distribution coefficients?
A: Ratios that describe how a solute distributes itself between two immiscible phases, influencing separation during extraction.
Q: Why is continuous extraction advantageous?
A:
It enhances the mass transfer efficiency of solutes,
allowing for better separation with less solvent
compared to batch extraction.
Q: What role does the stationary phase play in chromatography?
A:
The stationary phase holds one phase in place
while allowing the mobile phase to move past it,
facilitating the separation of solutes based on their interactions.
Q: What is meant by "theoretical plates" in chromatography?
A:
Sections in the chromatographic column where:
Equilibrium is assumed to be achieved.
Contributes to efficiency and resolution of the separation process.
Q: What is the purpose of a solvent in solvent extraction?
A:
Transfers solutes from one liquid phase to another:
Isolating or purifying specific compounds.
Q: How does Gaussian distribution relate to analytical chemistry?
A:
Describes how solutions distribute around a mean value.
Essential for understanding the behaviour of solutes during:
Extraction and chromatography.
Q: What is the significance of solute partitioning in extraction processes?
A: Determines how effectively a solute can be:
Separated based on its affinity
for each of the two liquid phases involved in the extraction.
Q: What is the partition coefficient (K)?
A: An equilibrium constant that defines:
The ratio of a solute's concentration in the two immiscible phases at equilibrium.
Used to assess extraction efficiency.
Q: What does a higher partition coefficient indicate?
A:
Suggests that the solute has a greater affinity for the stationary phase compared to the mobile phase.
Leads to better extraction efficiency from the mobile phase.
Q: What is an immiscible liquid?
A:
Liquids that do not mix or dissolve in each other, such as oil and water.
Essential for solvent extraction processes.
Q: What is the meaning of "serial extraction"?
A: A process that involves:
Multiple stages of extraction.
Where solutes are extracted successively to improve separation and yield.
Q: How can you calculate the fraction (q) remaining at equilibrium in solvent extraction?
A:
Using the formula:
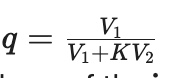
Where V1 is the volume of the initial phase and V2 is the volume of the other phase.
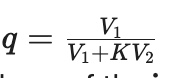
Q: What are the main components of a chromatography setup?
A:
A stationary phase.
A mobile phase (solvent).
A sample mixture to be separated.
Q: What is the function of the mobile phase in chromatography?
A:
To carry the sample through the stationary phase, facilitating:
The separation of components based on their interactions.
Q: How does the concept of equilibrium apply to solvent extraction?
A: Equilibrium is reached when:
The rate of solute transfer between the two phases is equal.
Allowing for a stable distribution defined by the partition coefficient.
Q: What types of compounds are commonly separated using solvent extraction?
A:
Organic compounds, pharmaceuticals, and metal ions.
Often based on their solubility in different solvents.
Q: What role does temperature play in solvent extraction?
A: Can affect:
The solubility of solutes in the solvents.
The partitioning behaviour, influencing extraction efficiency.
Q: What is retention time in chromatography?
A:
The time it takes for a solute to pass through the chromatographic system and reach the detector.
Used for identifying and quantifying components.
Q: What is the significance of the mobile phase's polarity in chromatography?
A: The polarity of the mobile phase affects:
How well different solutes interact with the stationary phase.
Thus influences their separation.
Q: Why is it important to choose the right solvent in extraction processes?
A: The solvent must:
Effectively dissolve the target solute.
Remain immiscible with the other phase to achieve efficient separation.
Q: What is the use of "end-capping" in chromatography?
A: A technique to improve the performance of the stationary phase by:
Reducing unwanted interactions.
Enhancing the separation of analytes.
Q: What are some common types of chromatography?
A:
Gas chromatography (GC)
Liquid chromatography (LC)
Thin-layer chromatography (TLC)
High-performance liquid chromatography (HPLC)
Q: What is the main principle behind gas chromatography?
A:
Separation of volatile compounds based on:
Vaporization of the compounds.
Interaction with a stationary phase.
Movement with a gas mobile phase.
Q: What is the function of a detector in chromatography?
A:
Identifies and quantifies separated analytes as they elute from the column.
Provides real-time data on:
Concentration of the analytes.
Retention time of the components.
Q: How is extraction efficiency typically improved in solvent extraction?
A: By optimizing parameters such as:
Solvent choice.
Temperature adjustments.
pH levels.
Number of extraction stages
Using techniques like:
Countercurrent extraction.
Q: What is the significance of baseline resolution in chromatography?
A: Ensures that peaks representing different compounds do not overlap.
Allows for:
Accurate identification of each component.
Quantification of each compound without interference.
Q: How does pH influence the distribution coefficient (D) in solvent extraction?
A:
Alters the ionization state of solutes.
Affects solubility in the aqueous phase.
Influences the distribution of solutes between phases.
Q: What is the role of affinity chromatography?
A: Separates biomolecules based on specific interactions such as:
Antigen-antibody binding.
Enzyme-substrate interactions.
Enables targeted purification of specific compounds.
Q: What are some challenges associated with solvent extraction?
A:
Proper selection of solvents.
Managing emulsions that may form during extraction.
Handling toxic or hazardous materials.
Ensuring complete phase separation.
Q: What does "selectivity" refer to in solvent extraction?
A:
The ability of a solvent to preferentially extract one solute over others.
Critical for achieving selective separations in complex mixtures.
Q: What impact does flow rate have on chromatographic separations?
A:
Affects interaction time of solutes with the stationary phase:
Too fast: May lead to poor resolution.
Too slow: Can lead to longer analysis times.
Q: Describe the concept of "sample matrix" in chromatography.
A:
The composition of the sample being analyzed.
Can influence the separation process.
May require method adaptations to mitigate matrix interference.
Q: What is the purpose of a mobile phase modifier in liquid chromatography?
A: Improves separation efficiency by altering:
Polarity of the mobile phase.
Viscosity of the mobile phase.
pH of the mobile phase.
Enhances interactions with the stationary phase.
Q: How does the choice of stationary phase affect the outcome of chromatography?
A:
Different stationary phases provide varying polarities and functionalities.
Can be tailored to enhance separation of specific analytes based on their properties.
Q: What is the importance of calibration in chromatography?
A:
Establishes response factors for analytes.
Ensures accurate quantification through comparison against known standards.
Q: How does adsorption chromatography differ from partition chromatography?
A:
Adsorption chromatography:
Separates analytes based on their ability to adhere to the stationary phase.
Partition chromatography:
Relies on differential solubility in liquid phases.
Q: What are some common applications of solvent extraction ?
A:
Recovery of essential oils.
Separation of metals.
Extraction of pharmaceuticals.
Purification of environmental samples.