Unit 6B Thermochem and Molar Heat
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Heat/Energy/Q
The energy transferred between systems or a system and its surroundings due to a temperature difference. It can be measured in joules and is involved in causing changes in temperature or phase.
Calorie
A unit of heat energy equal to the amount needed to raise the temperature of one gram of water by one degree Celsius.
Percent Energy Capture (%)
The percentage of energy that is successfully converted into useful work or stored in a system, compared to the total energy listed on the nutrition label multiplied by 100.
Endothermic
A process or reaction that absorbs heat from its surroundings, resulting in a decrease in temperature of the environment.
Exothermic
A process that releases heat energy to its surroundings, often occurring during a chemical or physical change.
dH (enthalpy)
A measure of the total heat content of a system, indicating the amount of energy absorbed or released during a process at constant pressure.
phase change
The transition of a substance from one state of matter to another, such as solid to liquid or liquid to gas, often involving energy exchange.
Molar Heat
The amount of heat required to raise the temperature of one mole of a substance by one degree Celsius, often used in the context of phase changes.
Endothermic Graph
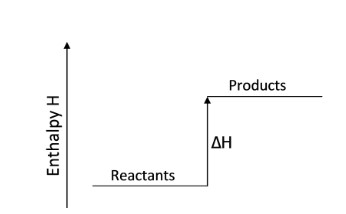
Exothermic Graph
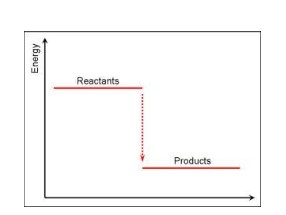
-dH (negative delta H)
A process or reaction where heat is released to the surroundings, resulting in a decrease in enthalpy. Feel hot, temperature will increase as the reaction releases energy to the surroundings.
+dH (positive Delta H)
A process or reaction where heat is absorbed from the surroundings, resulting in an increase in enthalpy. Feels Cold to the touch, temp of the surroundings will decrease as the reaction absorbs energy.