MCAT Gen Chem Review - Ch. 5+6: Chemical Kinetics; Equilibrium
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Chemical Kinetics
Gibbs Free Energy (Delta G)
Reaction Mechanisms
Mechanism
Intermediate
Rate-Determining Step
Molecular Basis of Chemical Reactions
Collision Theory of Chemical Kinetics
Activation energy Ea (energy barrier)
Arrhenius equation
Frequency factor
Attempt frequency
Ideal gas constant
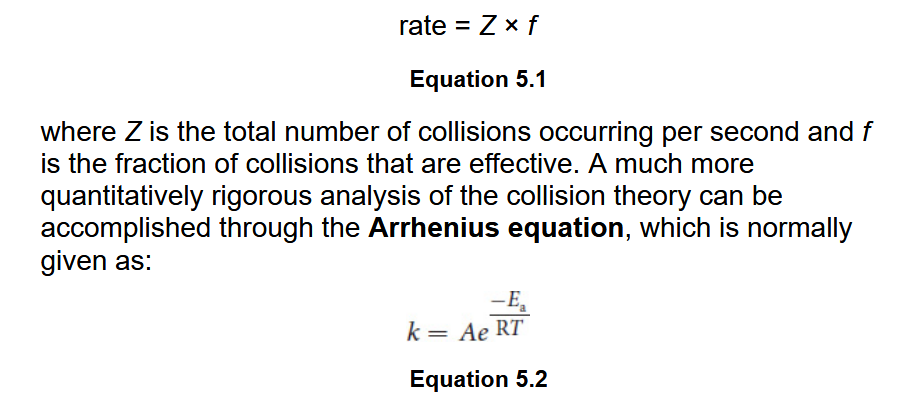
Transition State Theory
Reaction coordinate
Transition state
Activated complex
Free energy change of the reaction (Delta Grxn)
Exergonic vs. endergonic reactions
Factors Affecting Reaction Rate
Reaction Concentrations
Temperature
Medium
Catalysts
Homogenous catalysis
Heterogeneous catalysis
Definition of Rate
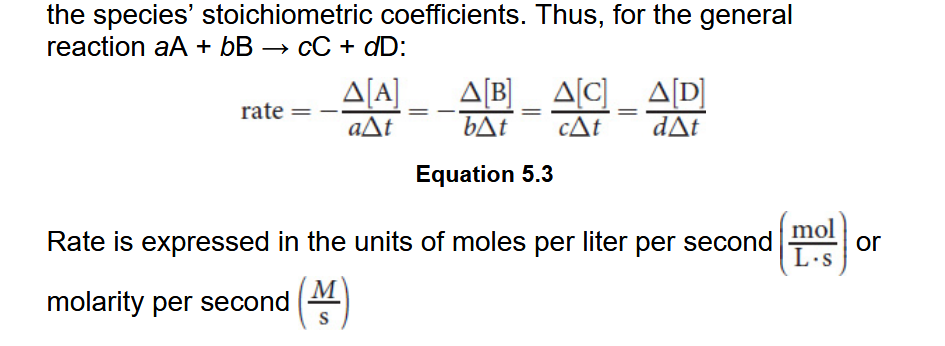
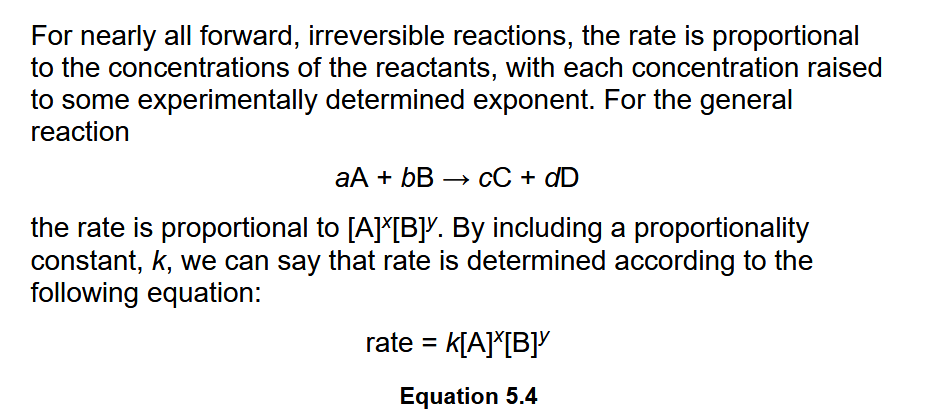
Determination of Rate Law
Rate Law
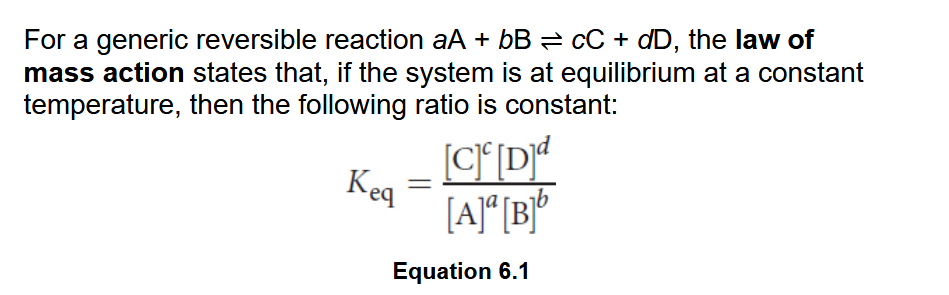
Law of Mass Action
Zero-order reaction
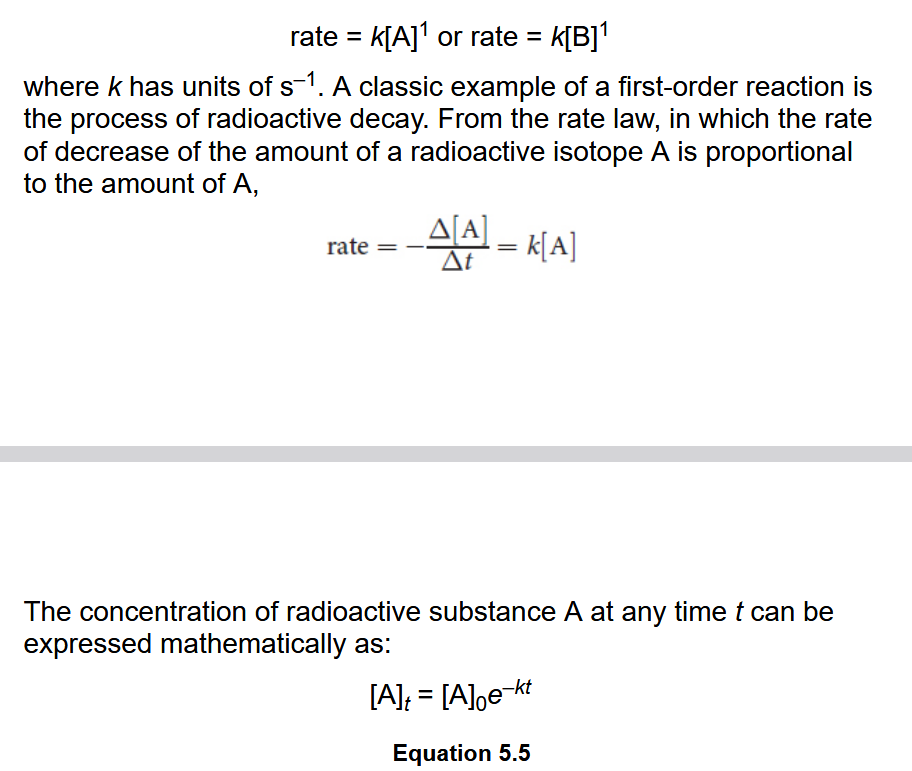
First-Order Reaction
Second-Order Reaction
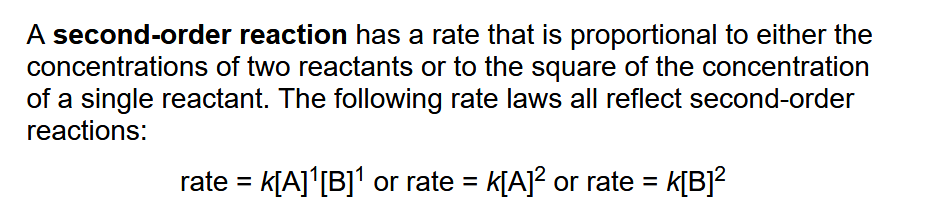
Mixed-Order Reactions
Broken-order
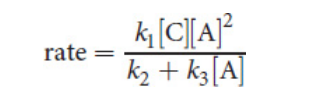
Dynamic Equilibria and Reversibility
Irreversible
Reversible
Dynamic equilibrium
Static equilibrium
Entropy
Law of Mass Action
Equilibrium constant
Reaction Quotient
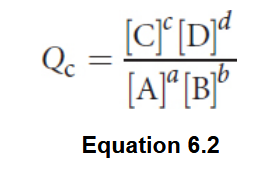
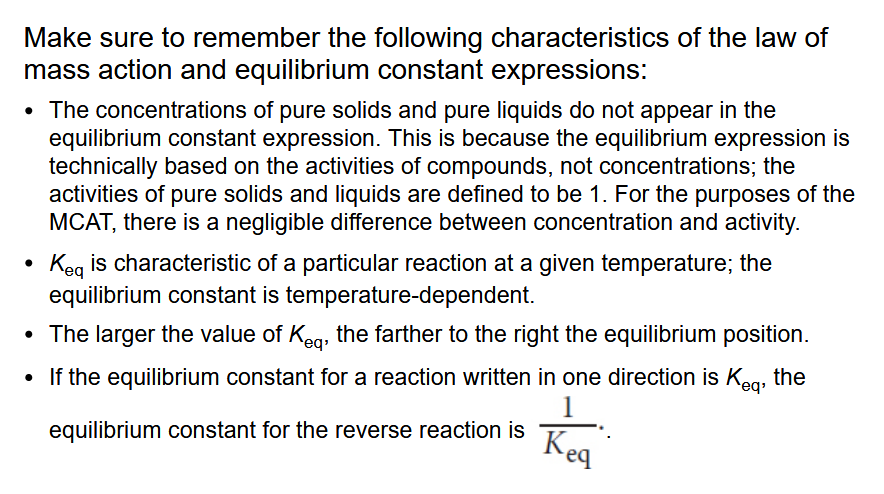
Properties of the Law of Mass Action
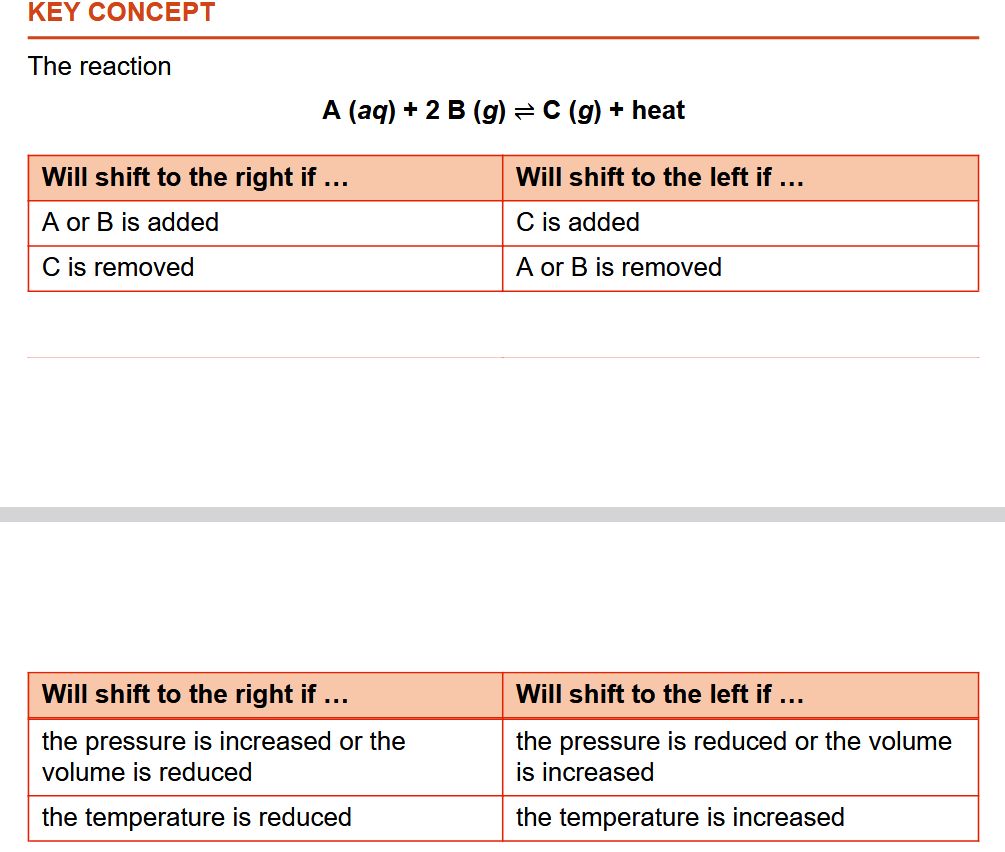
Le Chatelier’s Principle
Kinetic and Thermodynamic Control
Kinetic product
Thermodynamic product