IMED1002 - Macromolecules
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Virus
- not cells, but contain genetic material
Basic structure:
- Outer protein coat: capsid
- Inner core of nucleic acid: RNA/DNA Tail
- many have an envelope - lipid bilayer with proteins
Macromolecules
- polymers (chains) of single units (monomers) linked by covalent bonds
- 4 main types (with monomer):
- Nucleic acid (DNA, RNA): monomer is nucleotides
- Lipids/Fats: monomer is fatty acids
- Proteins: monomer is amino acids
- Carbohydrates: monomer is monosaccharides
Carbohydrate
- General Formula: Cn H2n On (where n=3-8)
- Monomer: monosaccharide
Oligosaccharides
- 3-10 monosaccharides
- Glycosidic Bond: covalent bond between hydroxyl of a sugar and the hydroxyl of a second sugar (or other alcohol containing molecule)
- disaccharides are simplest oligosaccharides. They are formed when two monosaccharides are linked by a glycosidic bond
- each monomer is termed a residue
Different Categories of Carbohydrates
CATEGORY NAME:
- 3 Carbons - triose
- 4 carbons - tetrose
- 5 carbons - Pentose
- 6 Carbons - Hexose
FUNCTIONAL GROUPS:
- Aldehyde, Ketone etc.
NAMING:
- aldotriose - triose with an aldehyde
- ketotriose - triose with ketone
- aldohexose - hexose with aldehyde
- ketohexose - hexose with aldehyde
If it satisfies the formula Cn H2n On it is a carbohydrate
Chilarity
- when one half of a molecule does not match the other half, but has the same chemical structure
- Assignment of D or L
- Orientation of asymmetric carbon furthest from carbonyl group
- D sugar: Hydroxyl group on right
- L sugar: Hydroxyl group on left
Best way to tell if something is chiral: if all 4 bonds on the carbon have different substituents, and the order of these substituents is different on different carbons we have chilarity
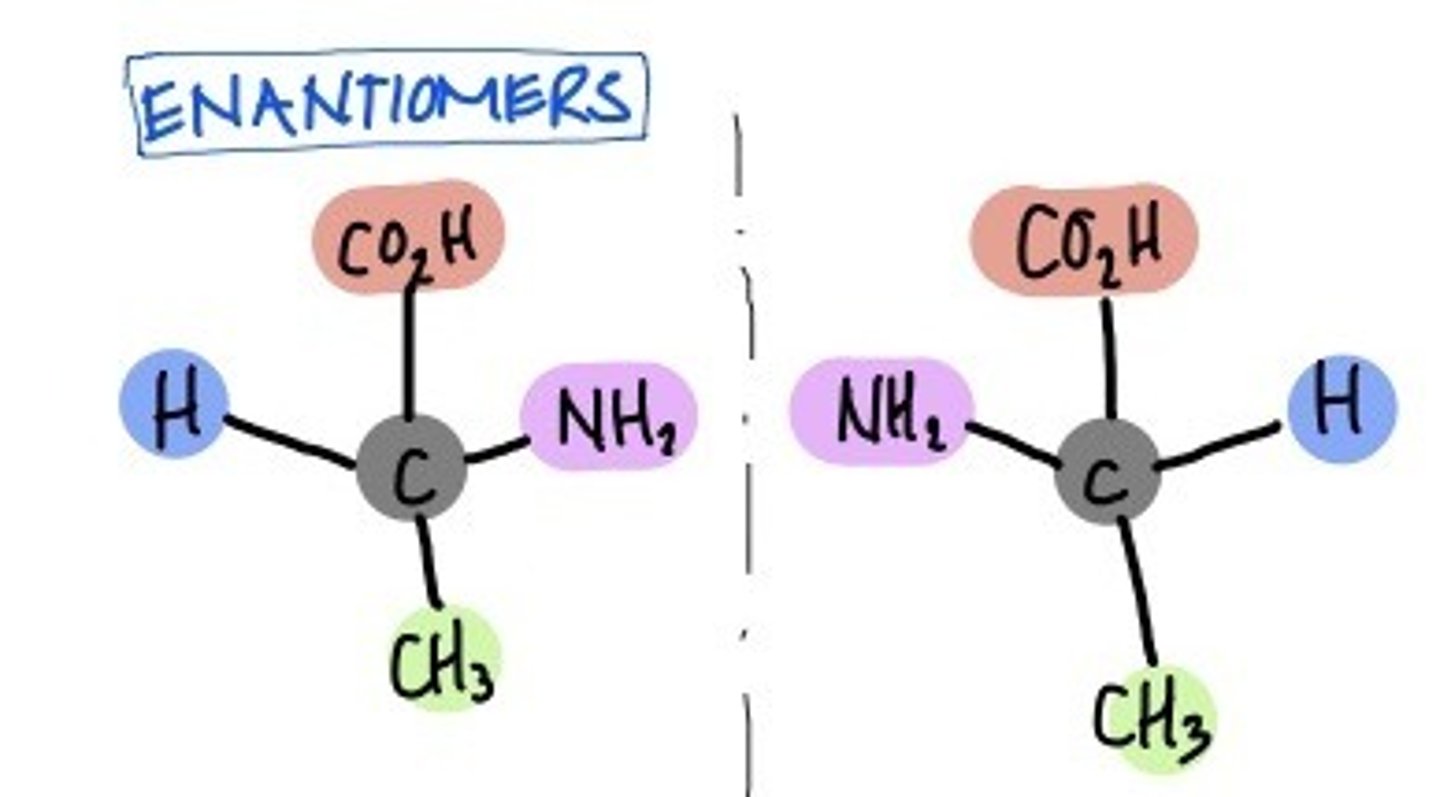
Enantiomers
isomers that are mirror images of each other. E.g search on google for D-erythrose and L-erythrose
Diastereoisomers
stereoisomers that are not mirror images of each other. Slide 14 of Lecture 2 Macromolecules 2025 has a great example of this.
Naming Carbohydrate Chirality
- Carbons in sugars numbered from aldehyde carbon starting at 1 or from the carbon at the end closest to the keto carbon
-
Cyclic Forms of Carbohydrates
- 6 member ring: pyran
- 5 member ring: furan
Monosaccharide Derivatives
- variety of reactions produce derivatives of simple sugars, e.g sugar alcohol, sugar phosphate, deoxy sugars, amino sugars
Homopolysaccharide
a polysaccharide that contains only one kind of monosaccharide
- can be branched or unbranched
Heteropolysaccharide
a polymer made up of more than one type of monosaccharide. Can be branched or unbranched
Glycoprotein
A protein with one or more carbohydrates covalently attached to it.
Lipids
- organic molecules that are characterised by low solubility in water, thus are relatively hydrophobic
- Lipids that do not contain fatty acids: cholesterol
- Lipids that contain fatty acids (complex lipids)
- monomer: fatty acids
Functions of Lipids
- Storage of Energy: lots of available energy, good packing
- Insulation From Environment: low thermal conductivity
- Water Repellant: Hydrophobic nature keeps surface of organism dry
- Membrane structure
- Cofactors for enzymes
- Signalling molecules
- Pigments
- Antioxidants
Classification of Lipids
- lipids that contain fatty acids are further separated into triglycerides (storage lipids, neutral). These contain a backbone and chains of fatty acids.
- membrane lipids are polar (e.g phospholipids, glycolipids. These always have some kind of backbone e.g glycerol)
Storage Lipids
- Triglycerides
- Energy Rich: contains lots of C-C and C-H bonds
- Provide more energy then sugars
- serve as long term energy stores
- use carbohydrates for energy before use fats
- composed of two basic units: glycerol (polar) and fatty acids (long chain carboxylic acids, mostly nonpolar)
Saturated and Unsaturated Fatty Acids
- Saturated: no double bonds
- Unsaturated: contains C=C bonds
- Monounsaturated: 1 double bond
- Polyunsaturated: more then one double bond
- length and saturation determines MP/BP
Melting Point of Saturated vs Unsaturated Fatty Acids
- double bond means unsaturated fatty acids can not pack together efficiently as the entire molecule bends (cis and trans)
- saturated fatty acids have efficient packing
- therefore (usually) saturated fatty acids have a higher MP then unsaturated
Fatty Acids
- alkyl chain with terminal carboxyl group
- General formula: CH3-(CH2)n-COOH
Difference between cis and trans fatty acids
- trans C=C bond allows extended conformation
- Trans Fatty Acid form by partial dehydrogenation of unsaturated fatty acids (increases shelf life or stability at high temperature of oils used in cooking, also highly stable)
- consuming trans fats increase risk of cardiovascular disease
Nomenclature of Fatty Acids
- Number on left of ratio is number of carbons, right of ratio is the number of double bonds. Delta to the power of n shows the double bond is in the nth position from the carboxyl carbon
- e.g 16:1(Δ^9) is 16 carbons, one double bond in ninth position from carboxyl carbon
- e.g 18:2(Δ^9, 12) is 18 carbons, 2 double bonds. in 9th position and 12th position from carboxyl carbon
ALTERNATE CONVENTION FOR POLYUNSATURATED FATTY ACIDS:
- numbers C in the opposite direction. e.g for omega 3 fatty acid, it assigns C1 to methyl C (this is also designated w (omega symbol). The positions of the double bonds are indicated relative to the omega carbon.
Essential Nutrients
nutrients necessary for normal body functioning that must be obtained from food. e.g omega 3 fatty acids
Triacylglycerols
- fats and oils found in plants and animals
- also known as trigycerides
- 3 fatty acids joined to glycerol via condensation reactions (linked by ester bonds, esterified)
Phospholipids
- contain N and P as well as C, H and O
- contains glycerol phosphoric acid
- like oils, but one Fatty acid is replaced by phosphate
- phospholipids are amphiphillic (amphipathic) (contain both non-polar and polar regions)
Lipids that do not contain fatty acids
Steroids:
- regulatory molecules (composed of interconnceted rings, non-polar, hydrophobic)
- e.g vitamins, hormones, cholesterol
Cholesterol
- causes closer packing of lipids, enhances order and decreases membrane permeability
UP TO SLIDE 1 OF LECTURE 4 (AMINO ACIDS)
- L4 AMINO ACIDS