Biochemistry Chapter 3.3-3.4; Protein Structure
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
Do proteins need energy to fold into proper conformation?
No, they fold spontaneously and independently (no energy input).
What is primary protein structure?
The order/sequence of amino acids in a chain.
Changes to type, formation, and number of AA/s can alter function.
How do we read primary structure?
From N to C terminus (amino to carboxyl).
What is conservative substitution?
An alteration to primary structure; a single substitution of the same ‘category’ of AA.
Substitution of a;
hydrophobic VAL with LEU.
acidic ASP with GLU.
May not change conformation/function.
Do all alterations to primary structure affect function?
No, it depends on the magnitude and type of substitution.
AA’s with similar size and characteristics may have conservative affects.
AA’s with contrasting properties may severely alter conformation.
Deletion, insertion, multiples, etc.
Can primary structure tell us about overall shape?
No, the sequence of AA’s in a chain cannot tell us about the shape of the complex folds in a protein.
What properties define secondary structure?
The alpha helix and beta sheet.
Coils, turns, and loops.
What is the alpha helix?
The twisting of a single strand, formed by the peptide backbone turning into a right-handed, chiral spiral.
An intramolecular structure.
**Usually 10 AA’s per alpha helix.
How does hydrogen bonding stabilize the alpha helix?
The spiral is held together by a regular pattern of hydrogen bonds between carbonyl oxygen LP’s and the hydrogen of a peptide nitrogen.
The O and H are four amino acids away from each other.
O of C=O and H of N-H. The H is 4 AA’s in front of O.
H bonding is enabled due to resonance of the peptide bond, which creates dipoles.
What is “i+4” bonding?
The general name given to the hydrogen bonds that stabilize the alpha helix in secondary structure.
AA 1 is bound to AA 5, 2-6, 3-7…
Bonds are 4 AA’s apart; i+4.
How many AA’s does it take to complete one alpha helix turn?
3.6 AA residues.
360° in a circle = 100° of turn per AA.
What is the core of an alpha helix like?
The helix is coiled so tightly that there is no space within the core.
Almost solid, nothing can pass through.
Rod-like.
In secondary structure, where are side chains?
The arrangement of peptides and the tight coil mean side chains are only found on the outside of the helix.
How long is one alpha helix turn?
5.4 Å long per turn.
3.6 AA residues with ~1.5 Å per residue = 5.4 Å total.
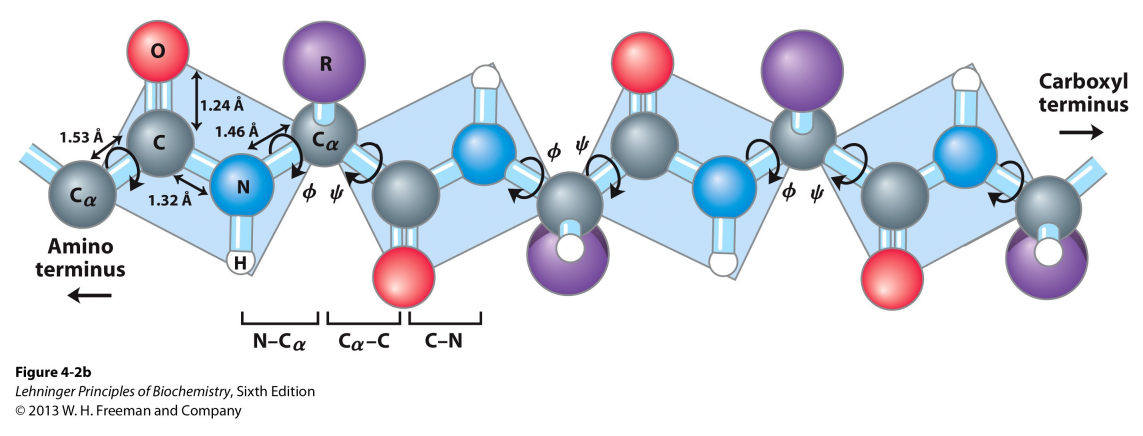
What are dihedral angles?
They are areas of rotation between two planes of atoms, on either side of the Cα. They stabilize secondary structure by putting the backbone in spiral shape.
Peptide bonds are rigid and planar, this gives it flexibility.
Φ (phi): rotation about the N–Cα (amino carbon) bond.
ψ (psi): rotation about the Cα–C (carbonyl carbon) bond.
What are the common angles of phi and psi bonds and why can’t they be any value?
Some bond angles are unfavorable due to steric crowding, in secondary protein alpha helices, phi and psi bonds favor the formation of h-bonds.
Helix angles;
Φ (phi): -60°.
ψ (psi): -45°.
**Linear angles total to 180°!
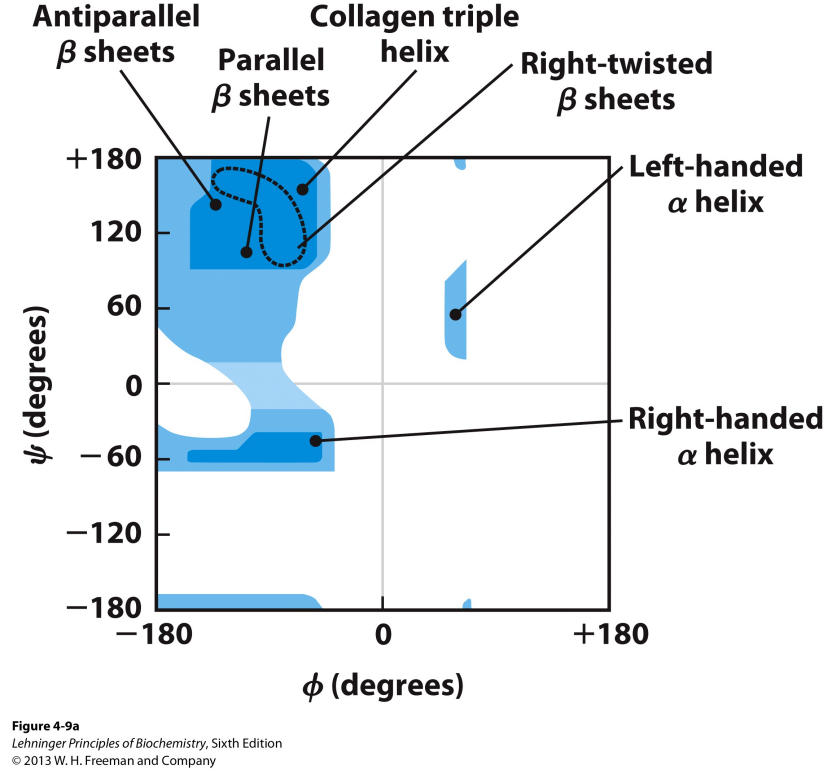
What is a Ramachandran plot?
It shows the distribution of Φ and ψ angles in secondary structure of a protein.
Shows that right-handed alpha helices favor -60→-45 formation for most AA’s.
What are the main forces/bonds that stabilize the alpha helix?
Hydrogen bonding between C=O and N-H on either side of a peptide bond.
i+4.
Dihedral angles favor h bonds and spriality (psi and phi).
Dipole-dipole from the single direction of carbonyl alignment.
Side chain interactions.
Glycine (GLY) alpha helix formation?
The hydrogen side chain discourages helix formation because it is floppy and has strong motional freedom.
Discourages optimal dihedral bond angles.
Proline (PRO) alpha helix formation?
The side chain link prevents Φ (phi) and ψ (psi) angles from forming optimal geometry.
Kinks strand or terminates the helix at PRO.
What is the dipole moment in secondary alpha helix structure created by?
The helix aligns carbonyl groups in a single direction, stacked.
“Macrodipole’
Partial positive on N-terminus.
Partial negative on C-terminus.
What is a beta sheet?
It is the other type of secondary structure in proteins, opposite of alpha helices.
They are stretched out polypeptides fixed together in the same plane as the sheet.

What forms the beta sheet? What is the structure?
They can be formed by numerous extended polypeptide chains, through hydrogen bonds.
The carbonyl O and amine H of two different strands form an H-bond in the plane of the sheet → FLAT.
Each polypeptide has side chains in an alternating arrangement, perpendicular to the plane of the sheet (90 degrees).
What is a parallel beta sheet? How does it affect H-bond formation?
The strands forming the sheet run in the same direction; both strands have C and N terminals on the same side.
Rectangular pattern of hydrogen bonds.
What is an antiparallel beta sheet? How does it affect H-bond formation?
The strands forming the sheet run in opposite directions; the C and N terminals of polypeptides are on opposite sides.
Trapezoidal shape of hydrogen bonds.
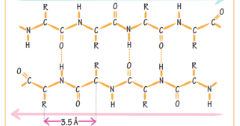
What type of beta sheet is this?
Antiparallel beta sheet.
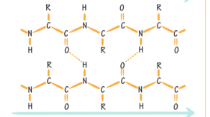
What type of beta sheet is this?
Parallel beta sheet.
How do alpha helices and beta sheets connect to eachother?
Through hairpin turns, loops and coils.
9 types of hairpin turns, based on phi and psi angles.
Each of 3-4 AA’s.
Loops and coils are longer but don’t contain a or b elements.
Link a and b but don’t contain them.
What stabilizes secondary structure?
H bonding between the backbone.
What is tertiary protein structure?
Provides the overall shape of a single protein.
How helices and beta sheets are joined to form higher order folds in a single polypeptide.
What is a motif?
Specific combinations of secondary structures; helices, turns, sheets, that appear repeatedly in a tertiary protein.
Part of a domain.
Help proteins fold in predoctable ways.
What are common motifs found in protein structures?
Alpha Motifs:
Helix-Turn-Helix
Three or Four-Helix Bundle
Globin Fold (8 helices).
Beta Motifs:
Greek Key (4 sheets)
B-Barrel
B-Propellor
Immunoglobulin (sandwich)
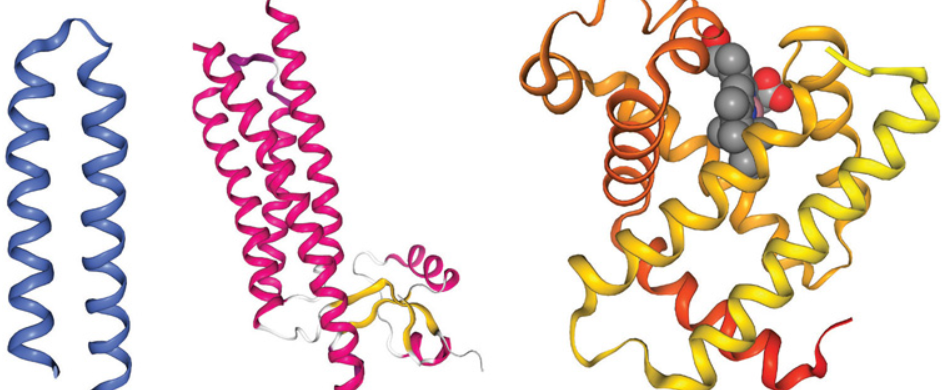
Label these motif’s.
Helix-Turn-Helix
Four Helix Bundle
Goblin Fold
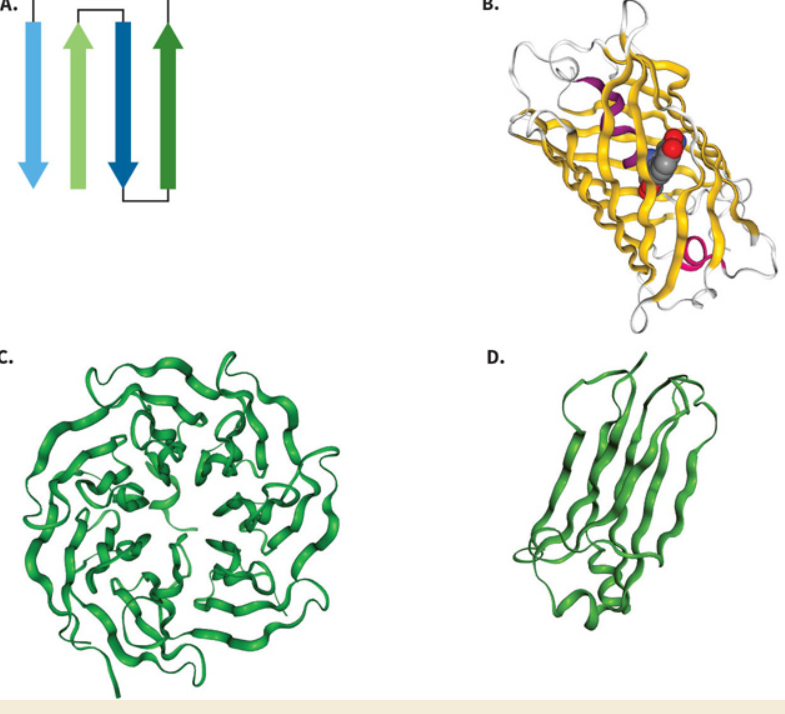
Label these motif’s.
Greek Key.
B-Barrel.
B-Propellor.
Immunoglobulin.
Are covalent or noncovalent forces the dominant forces that dictate tertiary structure?
While both are found, noncovalent forces dominate the assembly of protein structure, as there are more noncovalent interactions.
What covalent force is found in tertiary protein structure?
There is only one covalent (intramolecular) force aiding in tertiary structure; disulfide bonds.
The S-S linkage created by two oxidized cysteine (CYS) amino acid’s.
**Peptide bonds are covalent but don’t aid in tertiary structure.
What noncovalent forces are found in tertiary structure?
London dispersion; found in virtually all interactions.
Hydrogen bonds; between side chains with each other and the backbone.
In secondary, the H bonds are mainly within the backbone only.
Dipole-Dipoles; between polar side chains with each other and the backbone, as well as the macrodipole’s on alpha helices.
Salt-Bridging; positive and negatively charged AA’s interacting, described by Coulomb’s Law.
Cation-π Interactions; when basic AA’s (Kittens Happily Rise) interact with π bonds.
π bonds are in any double bond system.
As strong as H-bonds and as common as salt-bridges.
What is quaternary protein structure?
Complex proteins with subunits or multiple proteins.
Homotrimers.
Heterotrimers.
What is a fibrous protein?
These proteins have a long and extended shape, more sheet-like.
Used for structural support and protection; as collagen, keratin, etc.
Usually insoluble in water.
Usually have uniform types of secondary structure.
What is a globular protein?
These proteins are more compact and spherical/’glob-like’.
Used for function; as enzymes, hemoglobin, antibodies.
Usually soluble in water.
Usually have a mix of secondary, tertiary, quaternary structure.
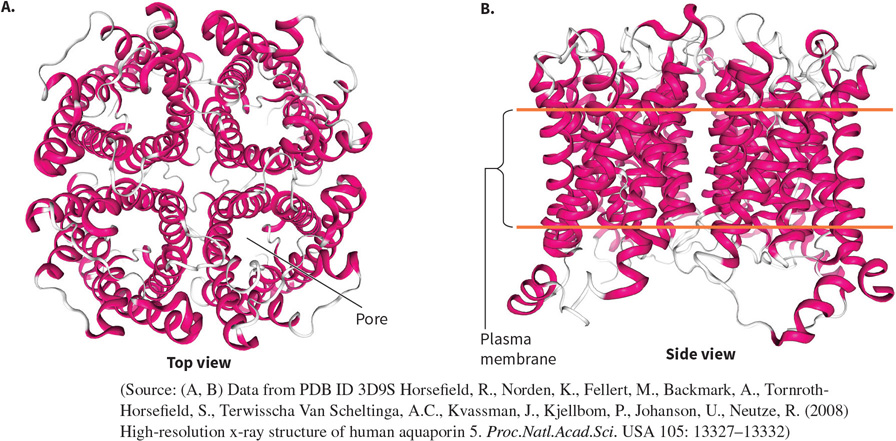
What protein is this? Structural and functional characteristics?
An Aquaporin - porous transmembrane proteins - a channel.
FUNCTION: to allow water in and out of cells.
Highly specific.
FOUND: in the plasma membrane (lipid bilayer, hydrophobic).
STRUCTURE: 4 subunits with six α helices bundled to create a quaternary protein. There is a hydrophilic pore and hydrophobic exterior within each subunit.
Homotetramer (quaternary).
Globular.

What protein is this? Structural and functional characteristics?
A Chymotrypsin - an enzyme.
FUNCTION: Cleaves dietary proteins into peptides (breaks down).
FOUND: secreted into the intestinal lumen.
STRUCTURE: 2 β -barrels and 1 α helix, with a small active site in its center.
Out of 245 AA’s, only 3 are used in catalysis.
242 are used as scaffolding to hold AA residues essential for regulation, localization, support, binding, etc.
Tertiary structure, cleaved.
Globular.
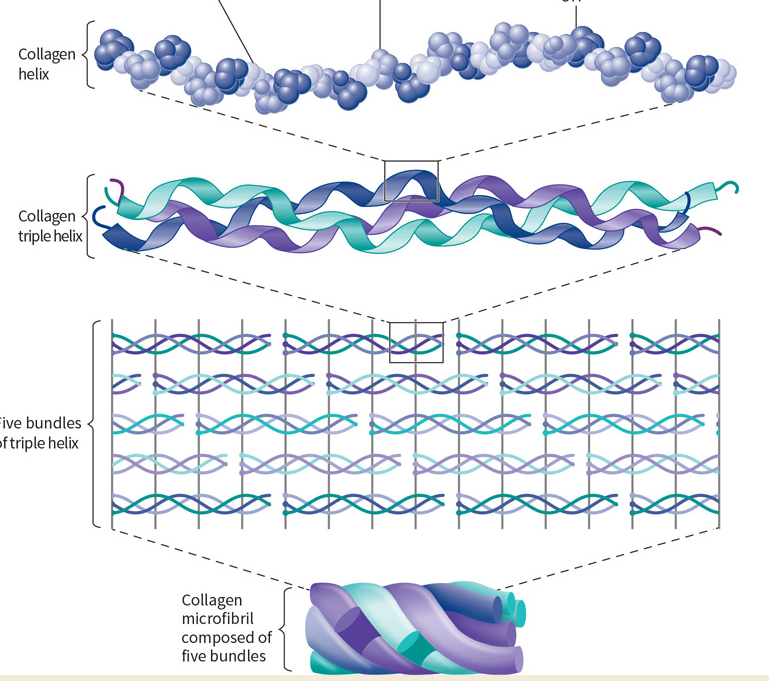
What protein is this? Structural and functional characteristics?
Collagen - a structural protein.
FUNCTION: provides strength, support and functional integrity to tissues.
FOUND: as isoforms (related and encoded by same genes) in organs, muscles, skin, bones, and tissues.
STRUCTURE: Individual molecules rich in GLY, PRO, and hydroxylated PRO.
Form left-handed collagen triple helices, and microfibrils (5).
Quaternary structure, many forms.
Fibrous.
What makes collagen unique? Explain its triple helix.
Collagen complexes are formed by polypeptides most abundant in GLY, PRO, and Hydroxyproline.
Three polypeptides twist to form a less compact, left-handed triple helix.
Stabilized by hydrogen bonds and steric interactions of GLY-X-Y pattern.
Glycine prevents steric clashes.
Proline and hydroxyproline use steric restrictions.
Resists stretching and allows tight packing.
Post-translationally modified by hydroxylation to provide extra stabilization and integrity.
An enzyme uses oxygen to add a hydroxyl to a proline.
Requires vitamin C to return the enzyme to its active form.
Lack of vitamin C = no hydroxylation = weakened connective tissues and Scurvy.
What is a collagen microfibril?
A bundle of five triple helices.
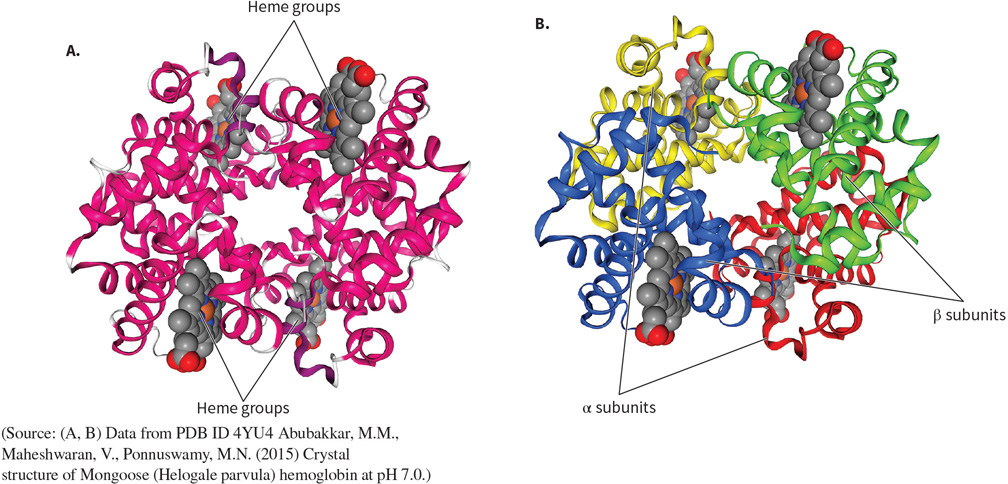
What protein is this? Structural and functional characteristics?
Hemoglobin - a transport protein.
FUNCTION: to transport oxygen around the body in blood.
FOUND: in RBC’s.
STRUCTURE: four subunits each made of 1 heme cofactor (heterocyclic ring), 8 a helices, and a central iron atom.
Tetrameric, quaternary structure.
Globular.
Alpha helices dominate.
How does hemoglobin transport oxygen? Affinity of Binding and Release?
One oxygen atom can bind to one heme through their iron core, so one hemoglobin can transport 4 oxygens.
The subunits communicate with one another, so affinity is altered — cooperative.
When Binding:
The binding of one oxygen increases affinity by manipulating the hemoglobin into a more ‘relaxed’ shape.
Makes it easier for the next oxygen to bind - positive cooperation.
When Releasing:
The release of one O2 molecule decreases affinity by manipulating the hemoglobin into a more ‘tense’ shape.
Makes it easier for the next oxygen to release - cooperative release.
What is allosteric regulation?
A protein’s activity/properties are controlled by molecules at sites other than the binding site.
How is hemoglobin transport an example of allosteric regulation?
When one O2 molecule binds to its site on one of the heme subunits, it influences hemes affinity for O2 at the other 3 subunits.
The binding of oxygen at one heme increases the affinity for oxygen at other hemes.
Positive homotropic allosteric regulator.
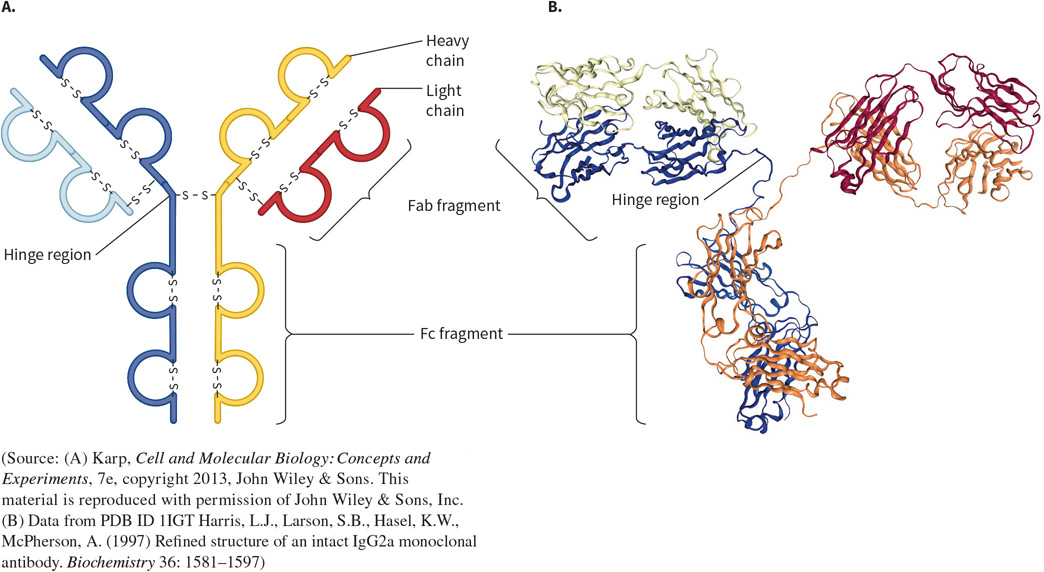
What protein is this? Structural and functional characteristics?
An Immunoglobulin (IgG) - a binding protein.
FUNCTION: part of the immune system, to recognize and bind to antigens.
FOUND: in the immune system.
STRUCTURE: They are diverse to be able to recognize and bind to numerous things. Contain a series of beta sheets held by disulfide bonds.
IgG; Four subunits (two heavy and two light chains).
Split into three fragments; Fab - two identical but variable fragments that bind to foreign molecule, and Fc - a constant fragment that binds to immune cells. They are bound by a hinge region for flexibility.
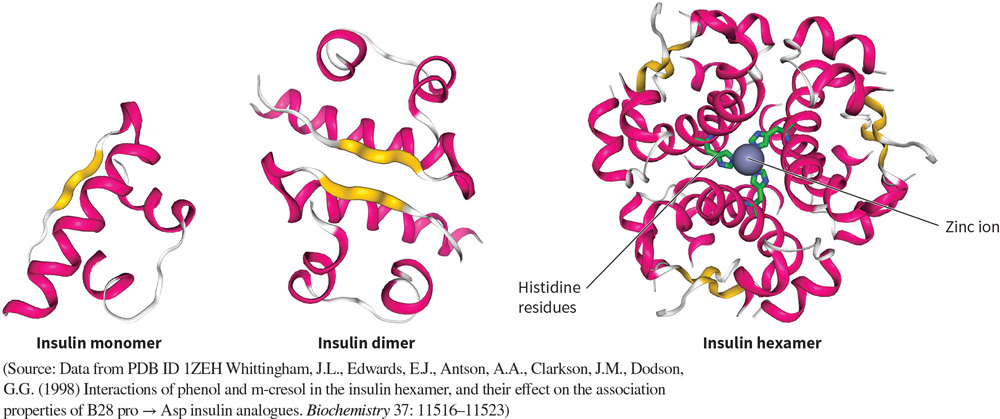
What protein is this? Structural and functional characteristics?
Insulin - a signaling protein.
FUNCTION: a growth factor that signals cells to store energy and absorb glucose.
FOUND: in the pancreas, bound to hormones.
STRUCTURE: A small protein made of 51 amino acids in alpha helical configurations, with a central zinc ion (cofactor).
Has monomers, dimers, and a hexamers, but only active in monomeric form.
Compare the structures of insulin being synthesized to its active form?
Insulin is synthesized in the pancreas as a single polypeptide chain of 104 AA’s.
The chain undergoes many rounds of cleavage in the ER to create a 51 AA protein made of two chains, held by disulfide bonds.
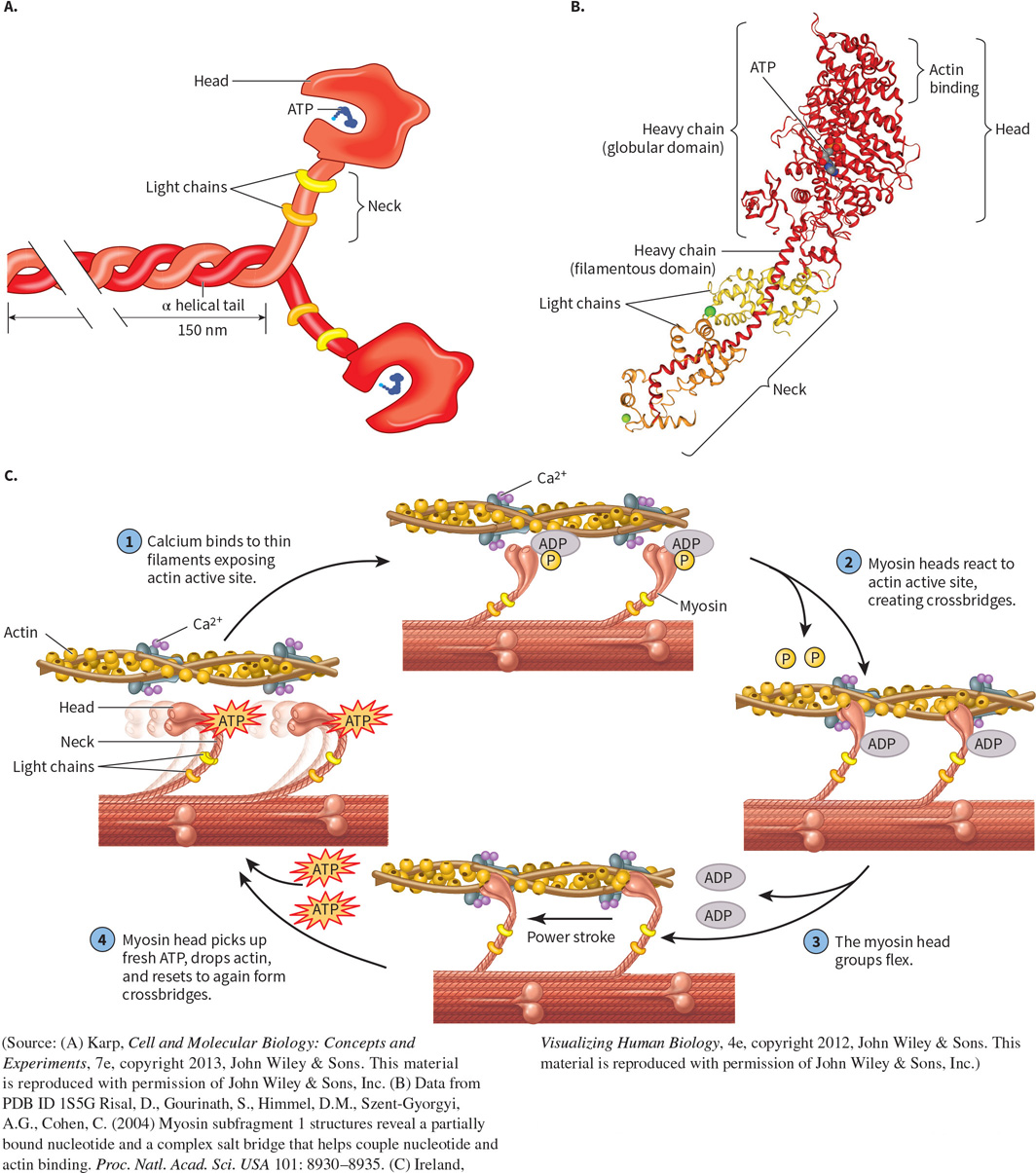
What protein is this? Structural and functional characteristics?
Myosin - a “molecular motor”, enzyme.
FUNCTION: uses ATP hydrolysis and interacts with actin to elicit muscle contraction.
FOUND: in muscle fibers.
STRUCTURE: has a globular head and filamentous tail made of heavy and light chains. Alpha helices dominate.
Heavy Chain;
Makes up the globular head, neck, and helical tail of myosin.
Light Chain;
Binds around the flexible neck for stability, reinforced by Calmodulin.
What does myosin interact with?
Actin filaments;
Myosin uses ATP hydrolysis to slide towards the positive end of actin filaments, thereby facilitating muscular contraction.
How does Myosin act as an enzyme?
It is an ATPase; catalyzes ATP hydrolysis to produce ADP, pi, and energy.
Occurs at the active site in the myosin head.
Converts chemical energy into mechanical work.
ATP binds to ATPase in the head — detaches from actin.
Myosin hydrolyzes ATP, cocking its head.
The head is rebound to actin at its cocked position.
The release of ATP products induces a powerstroke, pulling the filament.
Repetition produces muscle contraction or cell transport.
What is a ligand?
A molecule that binds to proteins
What binds a ligand to a protein binding site?
The noncovalent forces that make up the protein structure.
weak force.
What is the Bohr effect?
How changes in pH and [CO2] influence hemoglobin affinity for oxygen.
High [CO2] = low pH = low affinity.
It encourages T state formation.
2,3-BPG further stabilizes T state.
This ensures oxygen is released in areas with high metabolism (tissues, low pH), and is bound where O2 is low (lungs).
What does high CO2 do in terms of oxygen transport?
It lowers pH, lowering hemoglobin affinity.
It increases the Bohr effect by releasing H+.