Organic Chemistry Carbon Chains/Circles/Names
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
CH4
Methane
C2H6
Ethane
C3H8
Propane
C4H10
Butane
C5H12
Pentane
C6H14
Hexane
C7H16
Heptane
C8H18
Octane
C9H20
Nonane
C10H22
Decane
General Formula for an Open-Chain Alkane
C(N)H2(N)+2
Smallest Solid Alkane
C11H24
C4H10 Isomers
Butane and Isobutane
C8H12 Isomers
Pentane, Isopentane, and Neopentane
Naming Rules
1) Base Name on Longest All-Carbon Chain
2) Appendages on main chain are treated as substituents or substitutes for H's on the chain
3) Location of substituents are denoted by numbers
4) Number chain to give smaller number at first "point of difference"
5) Group identified substituents by name and show how many with numerical prefix.
6) Each substituent gets its own number (#,#)
7) Substituents of the same kind on different carbons are listed in increasing numerical order
8) Two "First Place" Substituents equidistant from the end of chain, minimize # of second substituent
9) Halogen Substituent Names and Alkyl substituents (Names on another)
10) Substituents of different kinds are alphabetized in the name (Numbering follows rule above)
11) Ignore Numerical Prefixes when Alphabetizing
12) If numbering is the same in both directions, give lower number alphabetically
13)If there are two long chains of equal length, choose the main chain with more but simpler substitutes.
Numerical Prefixes
1-mono
2-di
3-tri
4-tetra
5-penta
6-hexa
7-hepta
8-octa
9-nona
10-deca
Halogen substituents
F: flouro-
Cl: chloro-
Br: bromo-
I: iodo-
Alkyl Substituents
CH3: Methyl
CH3CH2: Ethyl
CH3CH2CH2: Propyl
Isopropyl
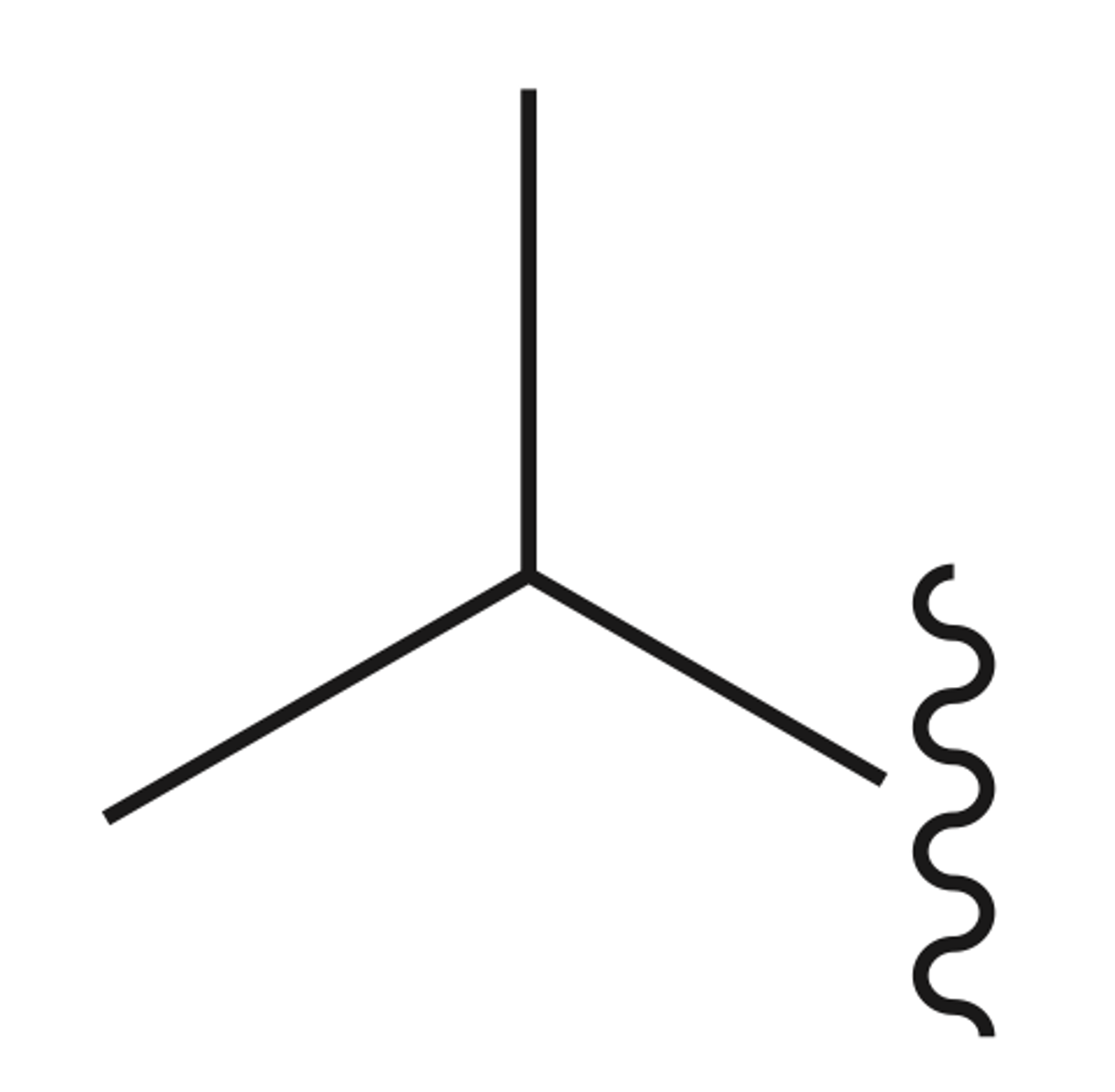
Isobutyl
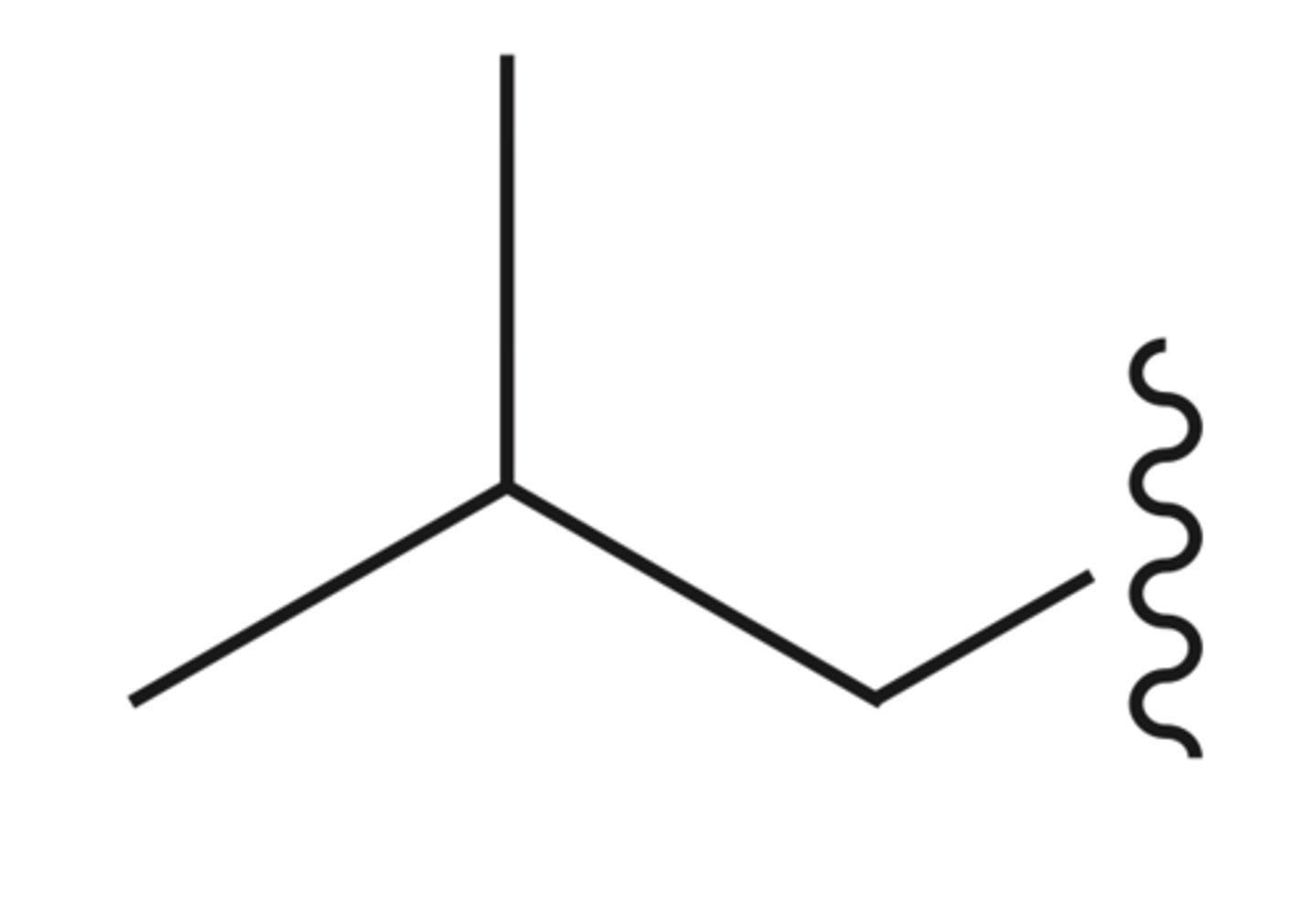
"sec"-butyl
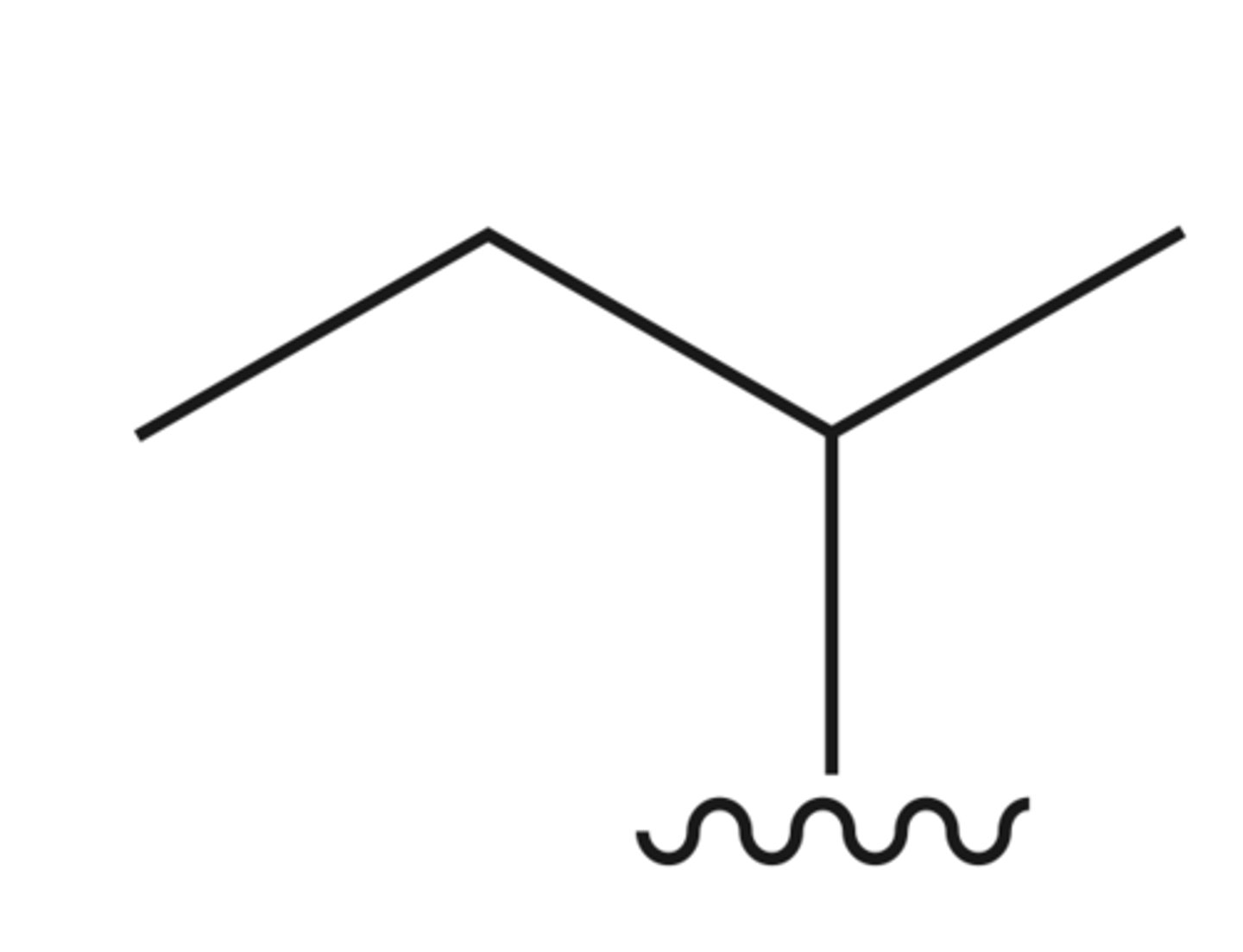
"tert"-butyl
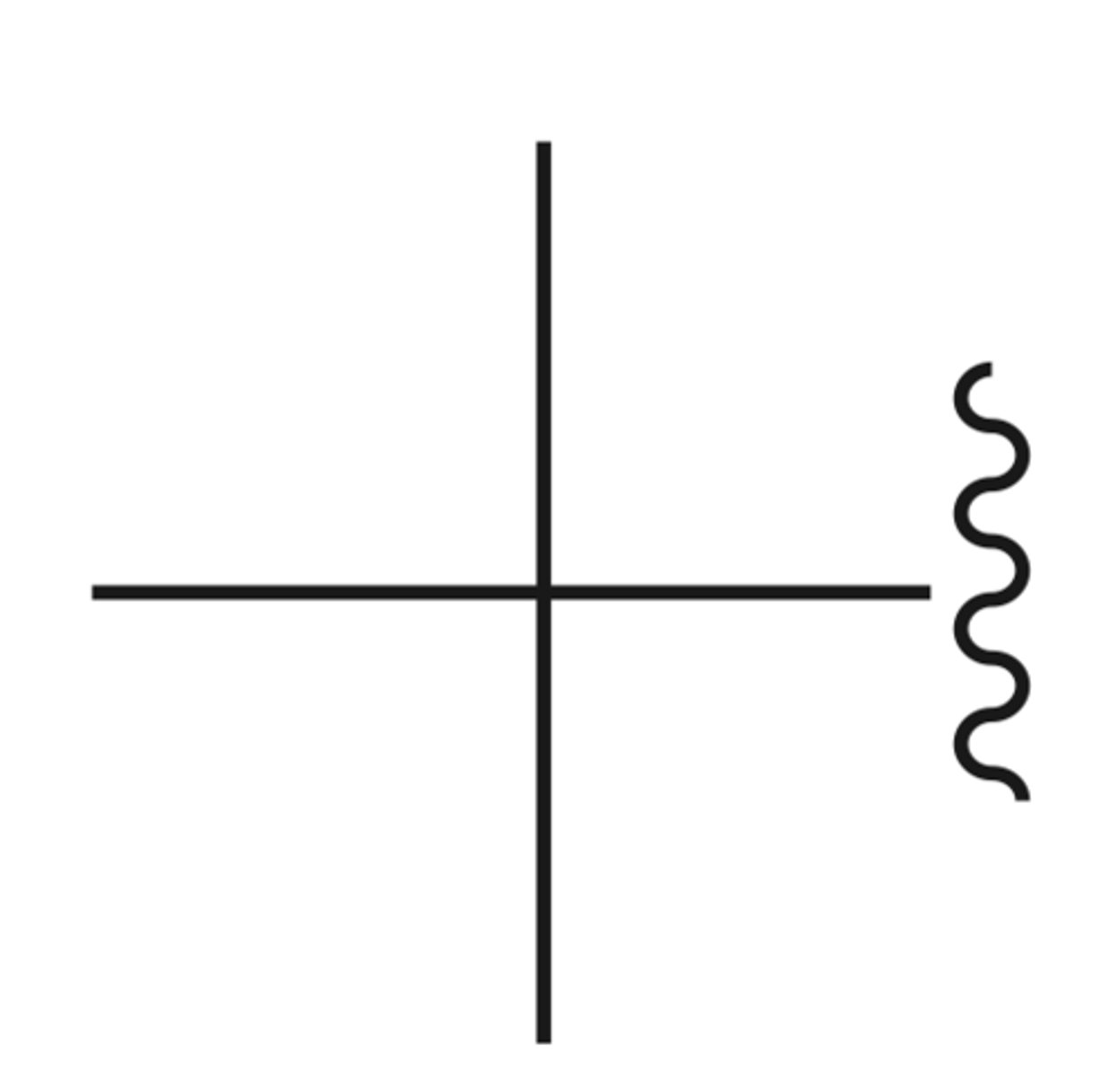
Neopentyl
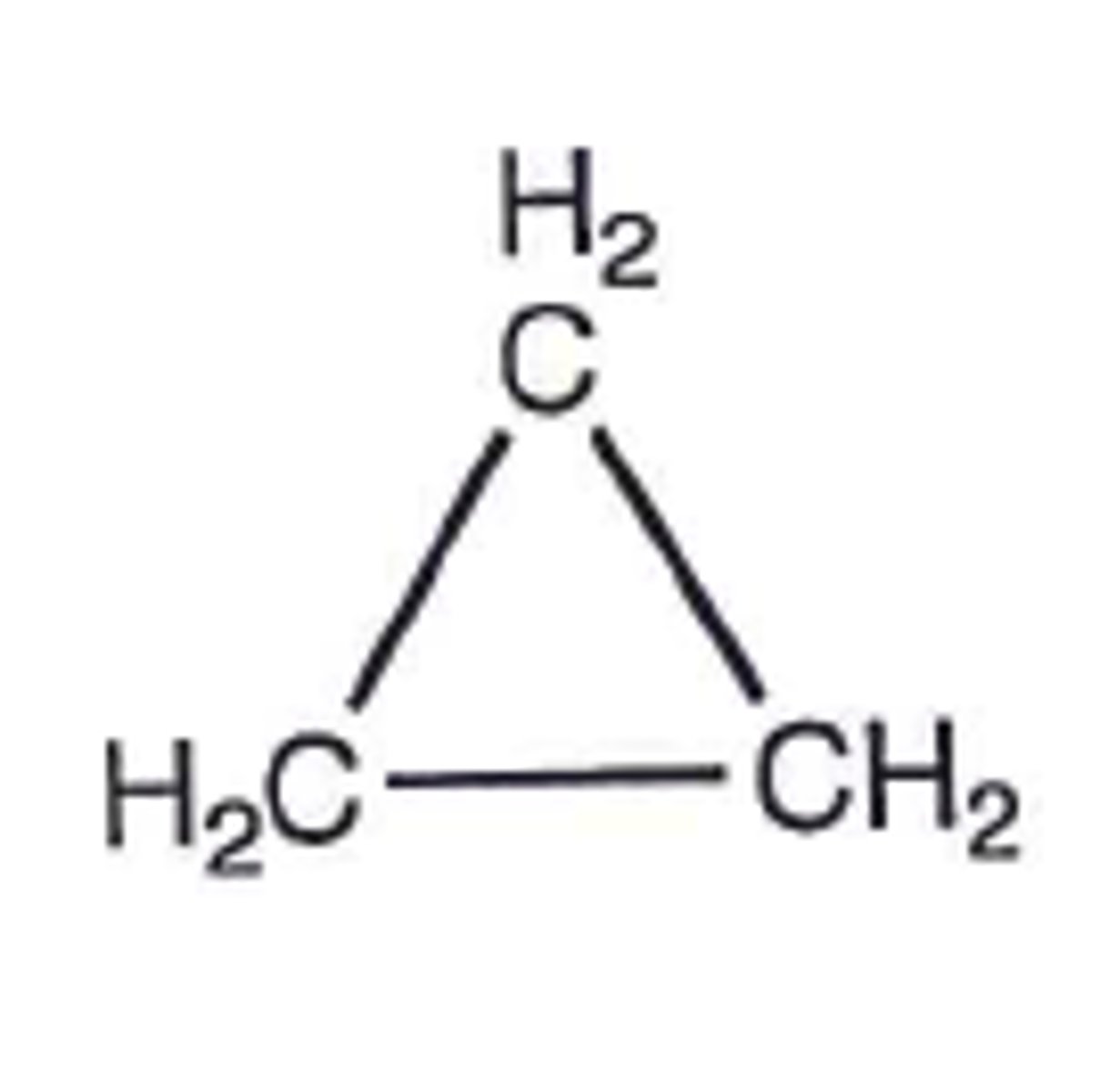
Cyclopropane
C3H6
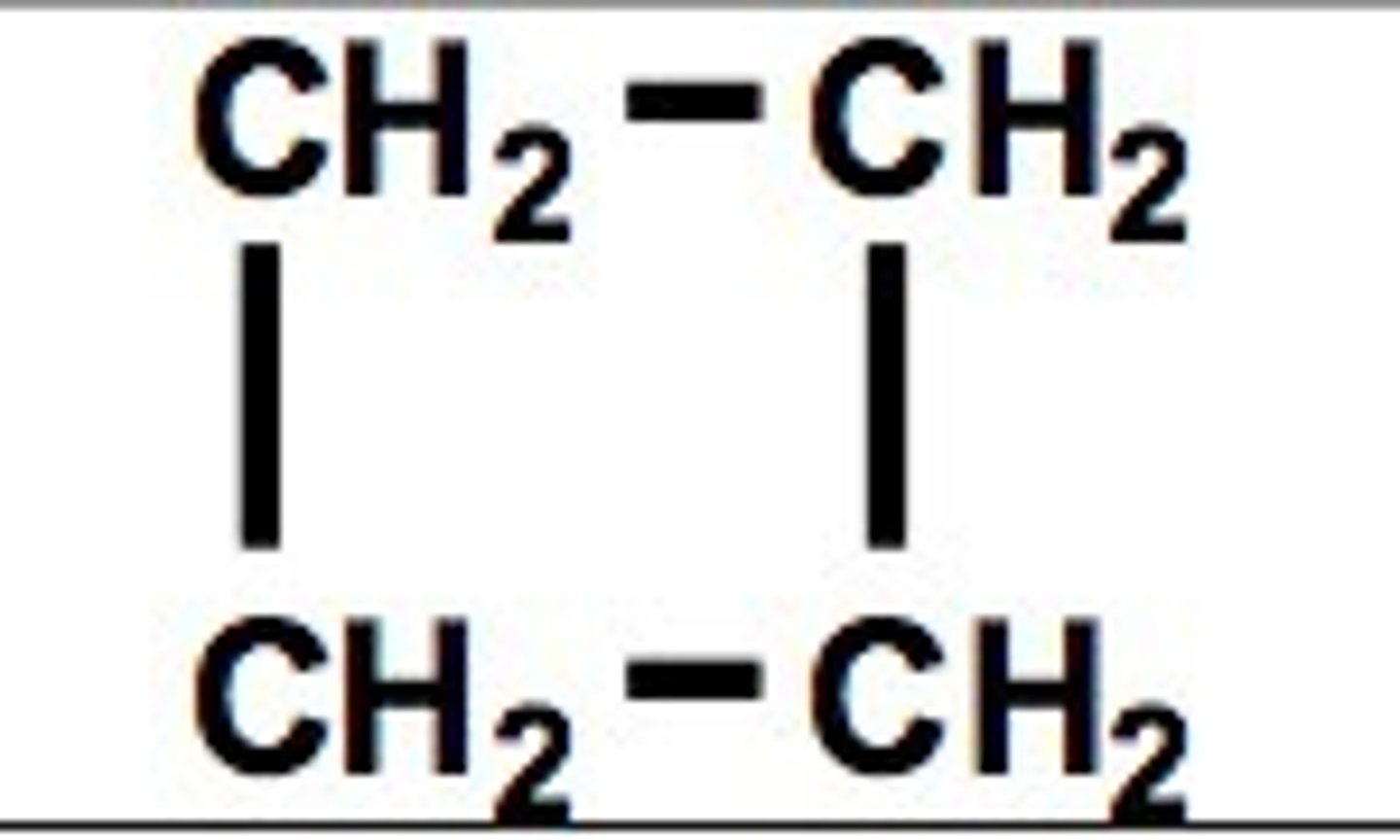
Cyclobutane
C4H8
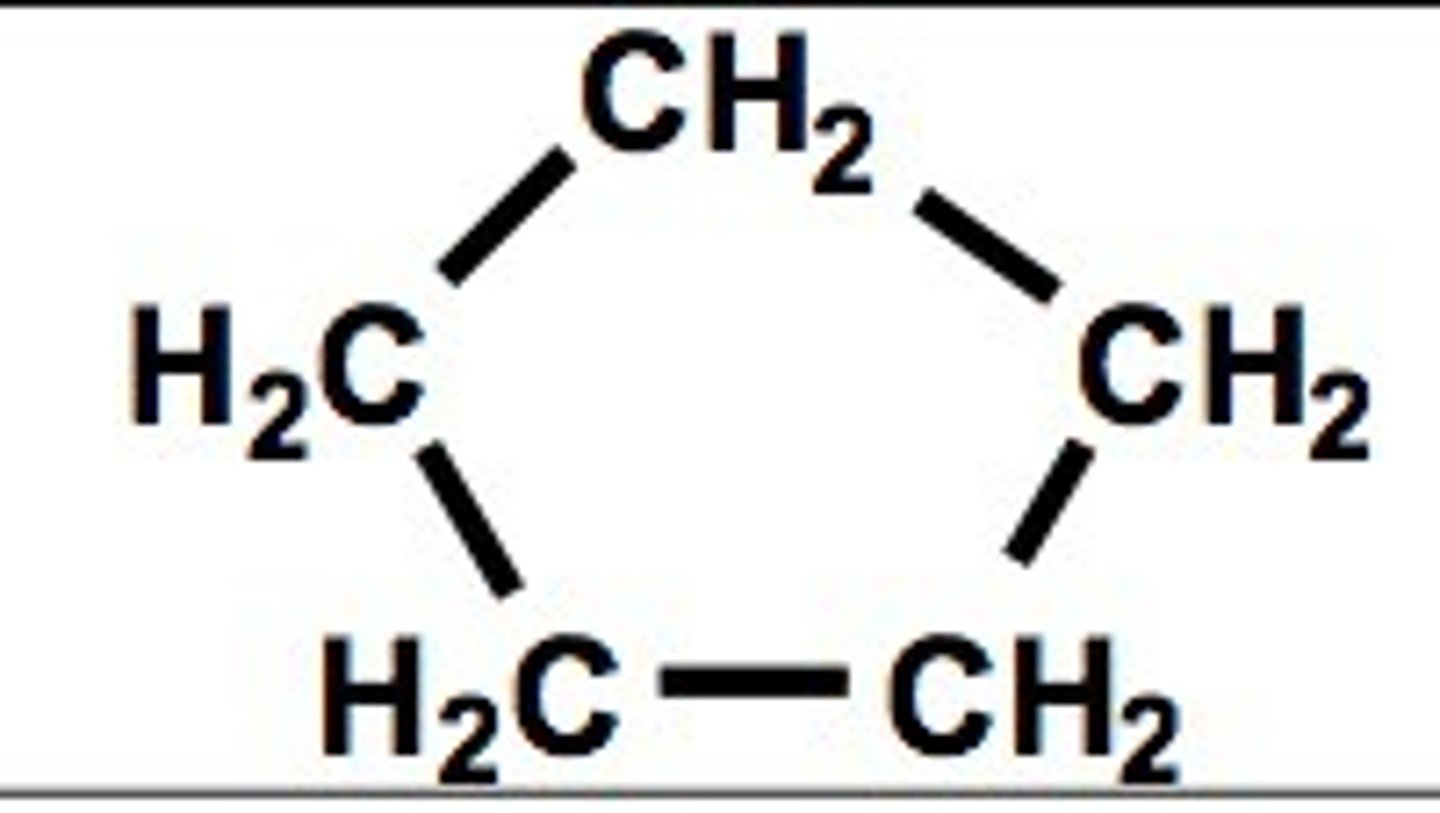
Cyclopentane
C5H10
Cyclohexane
C6H12
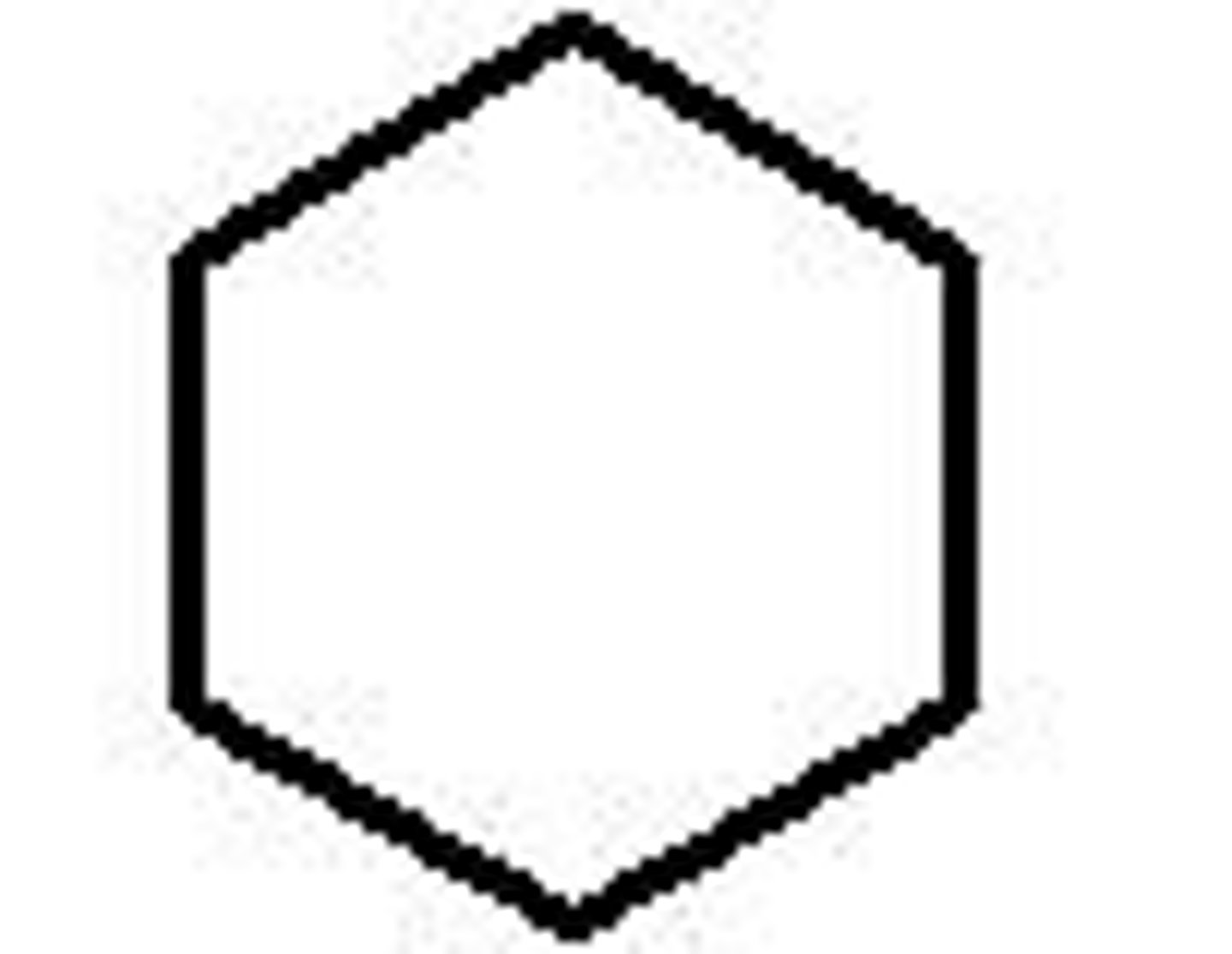
Cycloheptane
C7H14
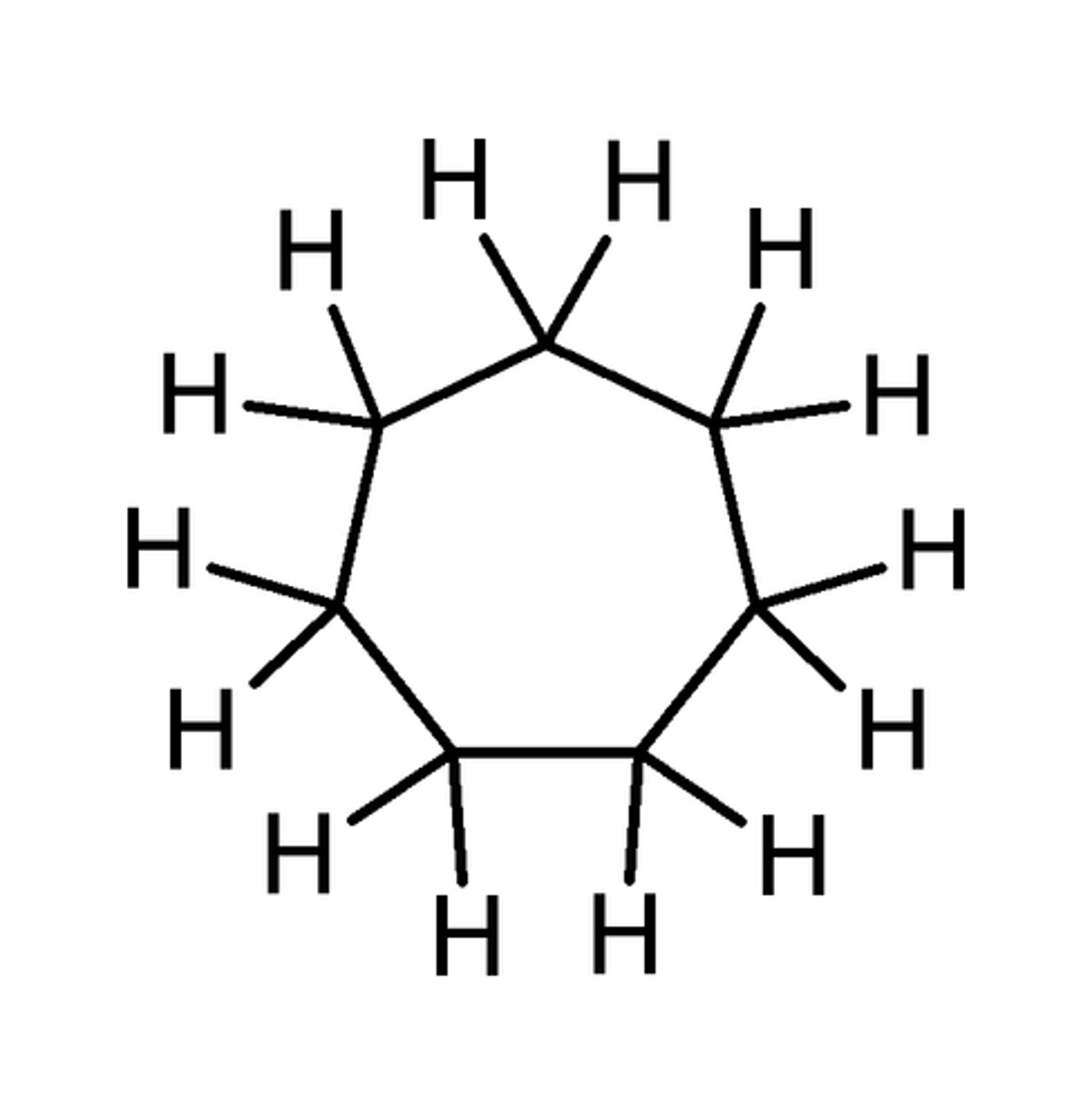
Cyclooctane
C8H16
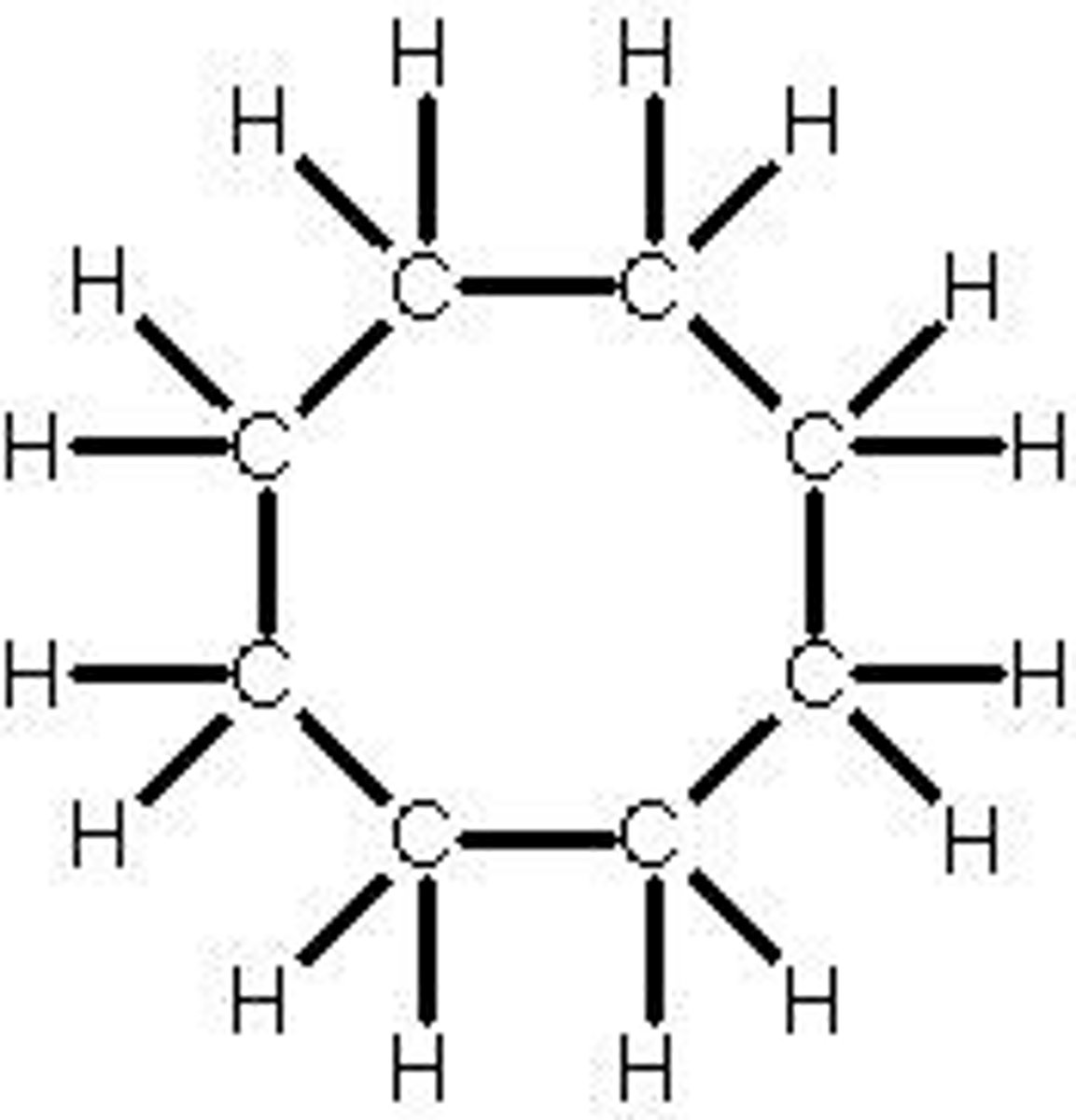
General formula for CycloAlkanes
CnH2n