Topic 7 - Energy changes
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
Why do chemical reactions occur?
So that elements can achcieve a more stable energy state, by gaining a full outer shell of electrons
How do chemical reactions take place?
By chemical bonding.
Where bonds are broken and new bonds are formed, the process involes the transfer of energy into and out of the reaction.
What are the terms used to describe the where the energy goes in and out of the reaction?
The system - is what happens in the chemical reaction
The surrounding - what haappens in the surroundings
Where does the energy in a reaction come from?
The chemical bonds
which are considered ars tiny stores of energy
What is used to measure the changes in heat energy?
A thermometer or calorimeter
What is the experiment to investigate the calorimetry of dissolving reactions? What are the results?
Add 2 spatulars of potassium sulphate to 15cm3 of water
Record the initial and final temperatures in degrees C
The temp deacreased - an endothermic reaction
What is the experiment to investigate the calorimetry of neutralisation reactions? What are the results?
Add 5cm3 of sodium hydroxide to 5cm3 of hydrochloric acid
The temperature increased - was an exothermic reaction
What is the experiment to investigate the calorimetry of acids + metal reactions? What are the results?
Add a strip of magnesium ribbon to 15cm3 of hydrochloric acid
The temperatture increased - was an exothermic reaction
What is the experiment to investigate the calorimetry of displacement reactions? What are the results?
Add approximately 2g of Zinc powder to 20cm3 of CuSO4
Temperature increased - was an exothermic reaction
What apparatus did you use to investigate the calorimetry of these rections?
Used:
polystyrene cup
polystyrene cover
Thermometer
In the experiment to investigate the calorimetry of a reaction why was a polystyrene cup used?
Because it is an isulator, so will insulate the reaction mixture and slows down the heat loss form the side and bottom
What is calorimetry ?
The process of measuring the amount of heat released or absorbed during a reaction
What is an exothermic reaction?
A reaction in which heat is lost to the surroundings and
Therefore the temperature of the reaction increases.
What is an endothermic reaction?
A chemical reaction where heat energy is gained from the environment and
Therefore the temperature decreases
Give an example of a reaction when salts dissolve in water.
State:
whether the product or reactants have more energy
Whether the temp increases or decreases
whether it is an exo or endo - thermic reaction
K2SO4 (s) → K2SO4(aq)
The products have more energy than the reactants
The temp decreasees
It is an endothermic reaction
Give an example of a neutralisation reaction.
State:
whether the product or reactants have more energy
whether the temp increases or deacreases
whether it is an exo or endo - thermic reaction
NaOH (aq) + HCl (aq) —> NaCl (aq) + H2O (l)
the reactants have more enrgy
The temp increases
An exothermic reaction
Give an example of a displacement reaction.
State:
whether the product or reactants have more energy
whether the temp increases or deacreases
whether it is an exo or endo - thermic reaction
CuSO4 (aq) + Zn(s) → ZnSO4 (aq) + Cu(s)
the reactants have more energy
Temp increases
Exothemic reaction
Give an example of a precipitation reaction.
State:
whether the product or reactants have more energy
whether the temp increases or deacreases
whether it is an exo or endo - thermic reaction
Ba(aq) + SO4 (aq) → BaSO4 (s)
products have more energy
Temp decreases
Exothermic reaction
When reactions take place in solutions, what is measured?
The change in heat energy (joules)
What is a energy level diagram?
Diagram used to represent the energy changes in reactions
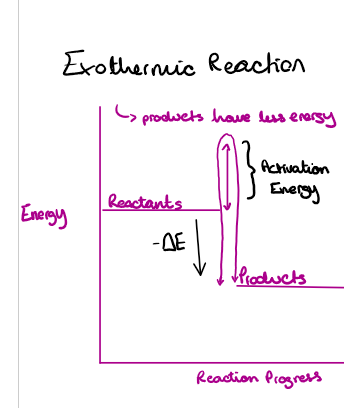
Draw the energy level diagram of an exothermic reation.
Explain what is going on
the reactants have more energy than the products
Negative change in energy
Temperature in the surrounding increases
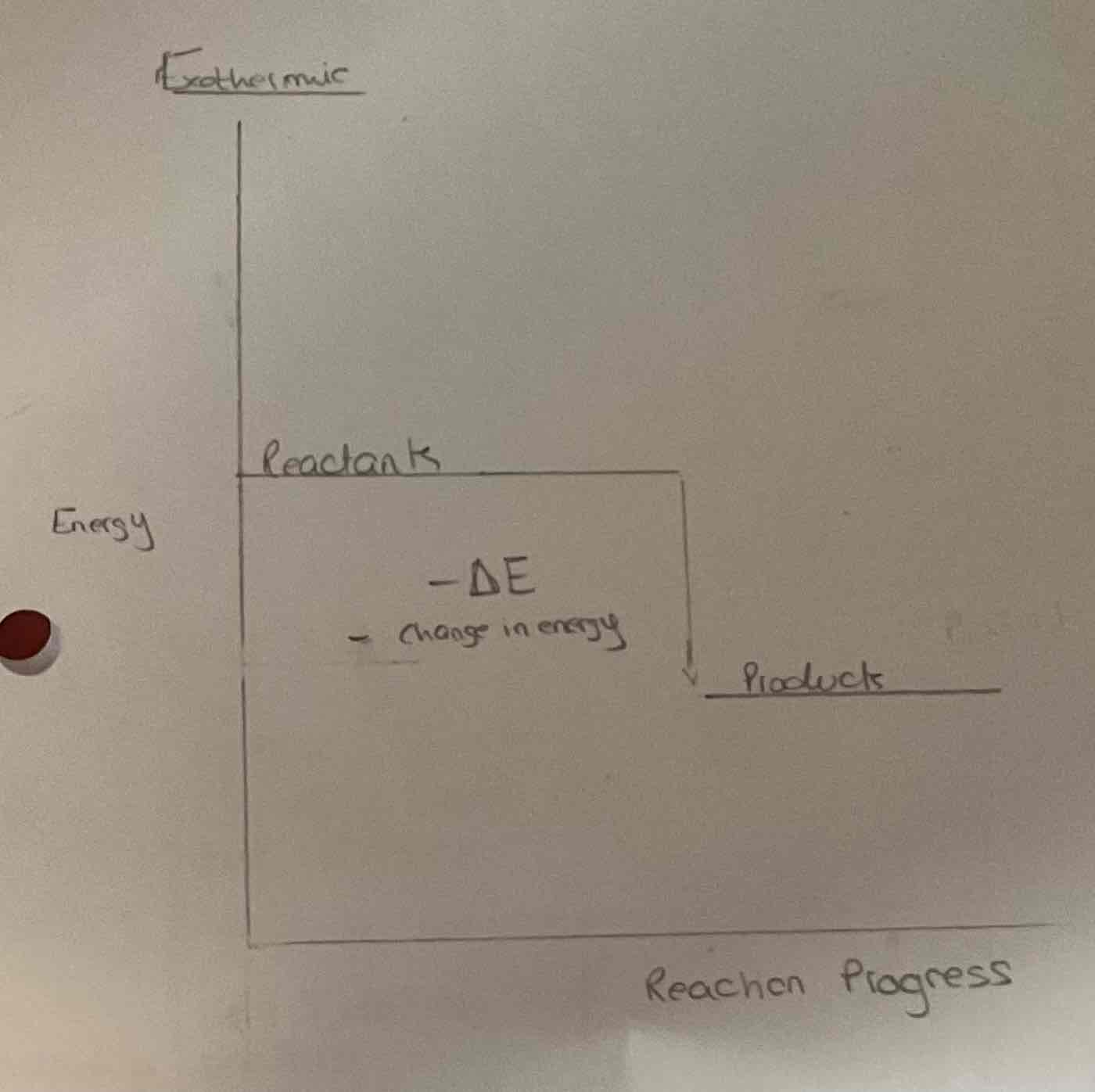
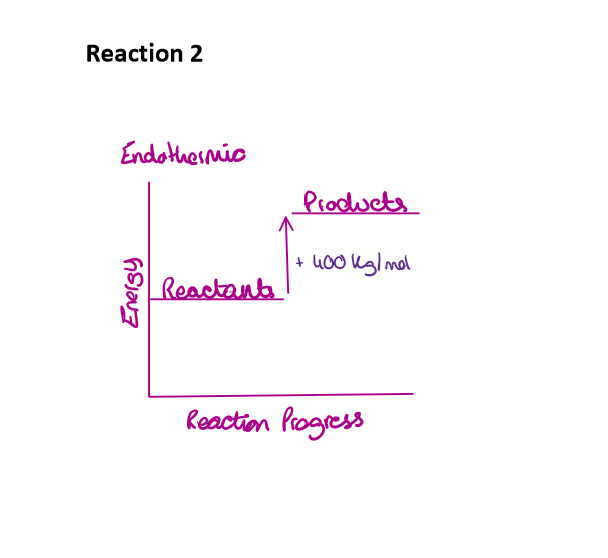
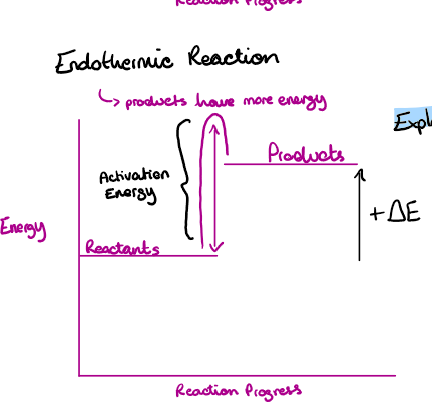
Draw the energy level diagram of an endothermic reaction
Explain what is going on
Reactants have less energy
Positive change in energy
Temperature in the environment decreases
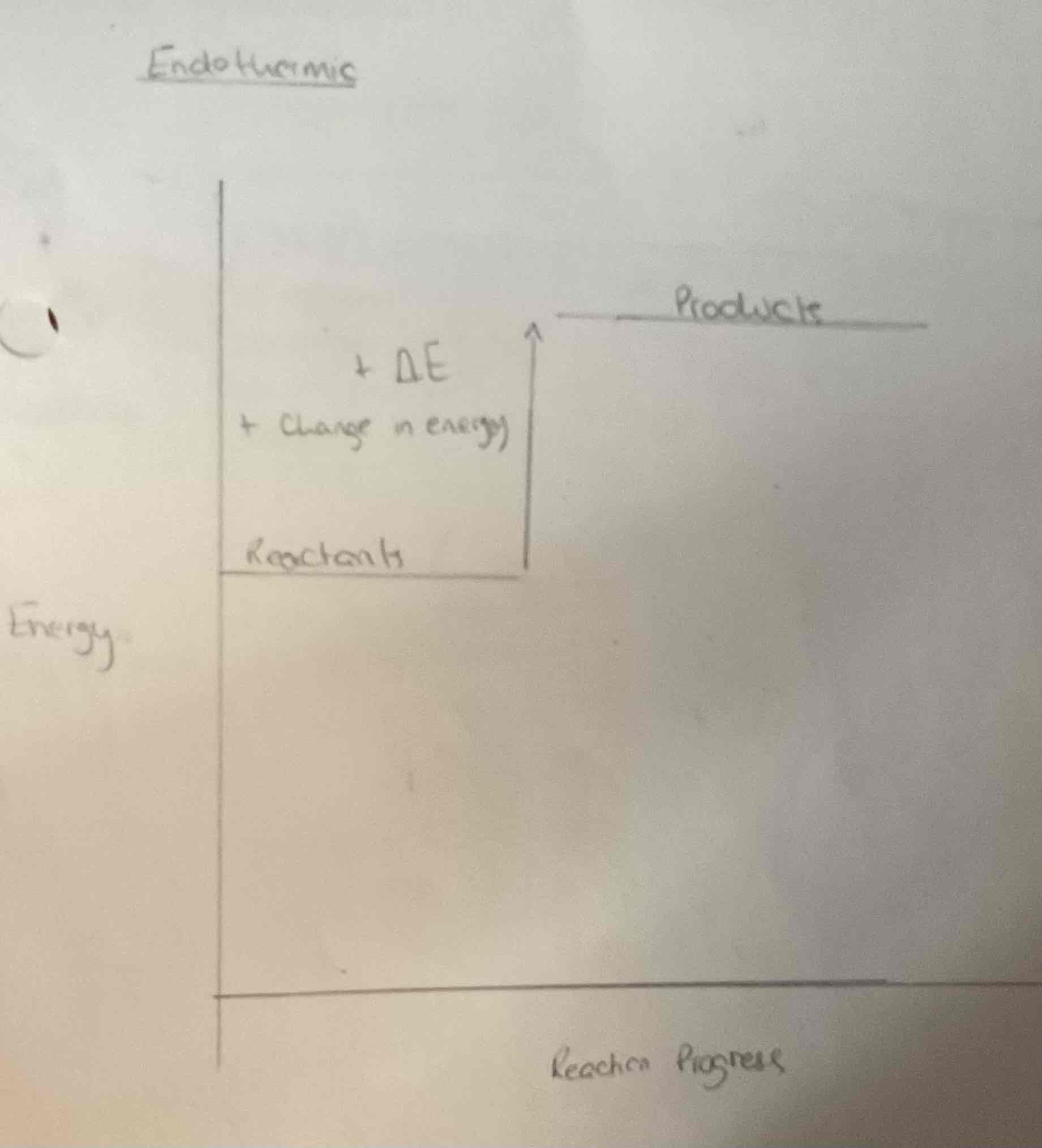

What doe this image represent?
The change in heat energy in a chemical reaction
In a chemical reaction what happens to the bonds in the products and the bonds in the reactants?
Bonds are broken down in the reactants
Bond are made in the products
What does the proccess of breaking bonds need?
What type of reaction does this make it?
Breaking bonds needs energy
therefore it is an endothermic reactio, this energy is absorbed form the environment
What does the process of breaking bonds do (in terms of energy)?
What type of reaction does this make it?
Making bonds releases enrgy
therefore it is an exothermic reaction as energy is rleased into the environment
What is the equation to calculate the change in energy?
Energy needed to break bonds - energy released whenbonds form
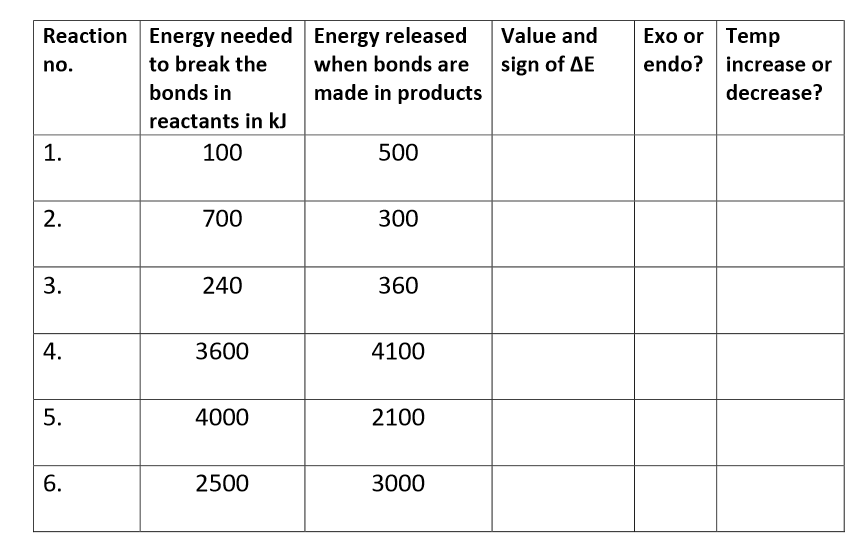
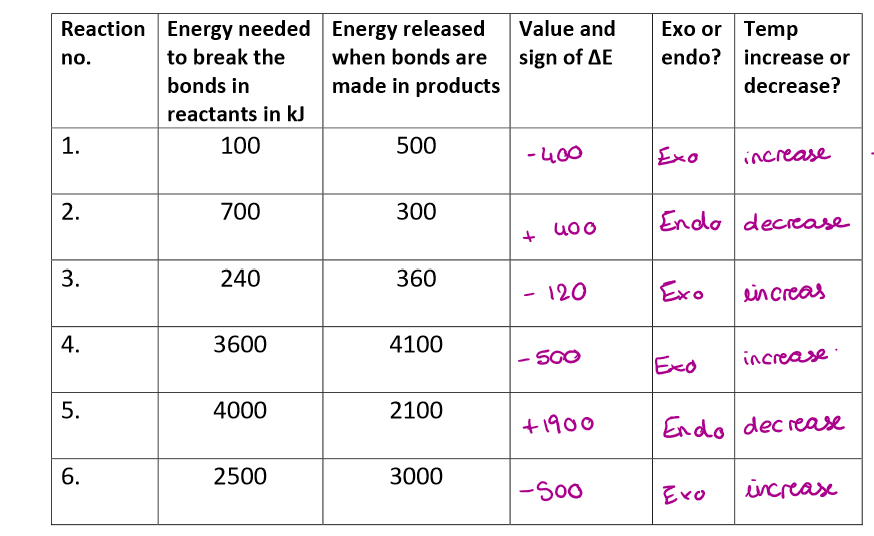
Complete this table

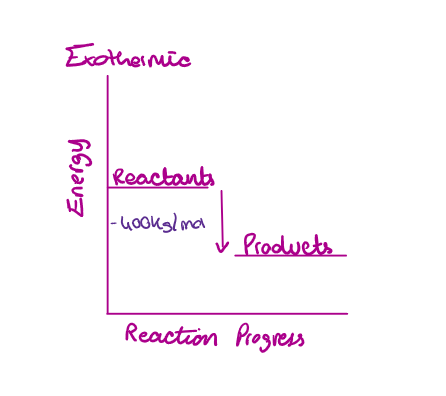
Fully draw and label an energy level diagram for reaction 1:

Fully draw and label an energy level diagram for reaction 2
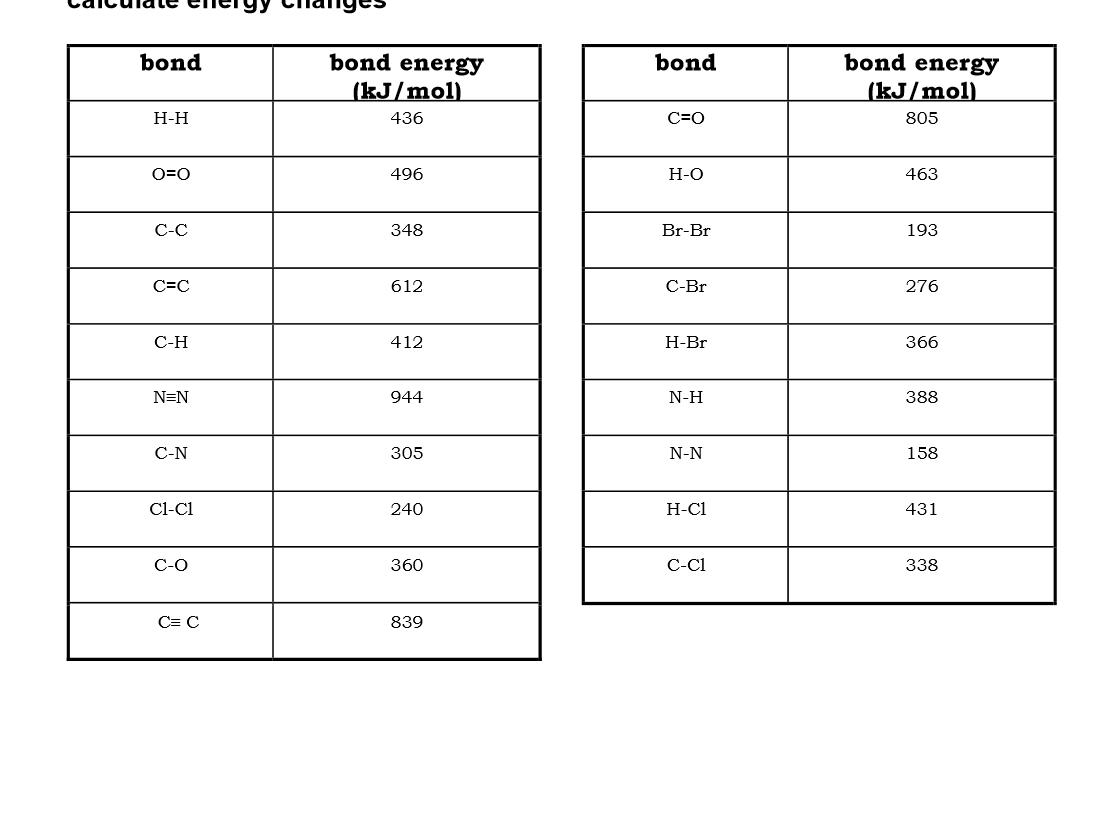
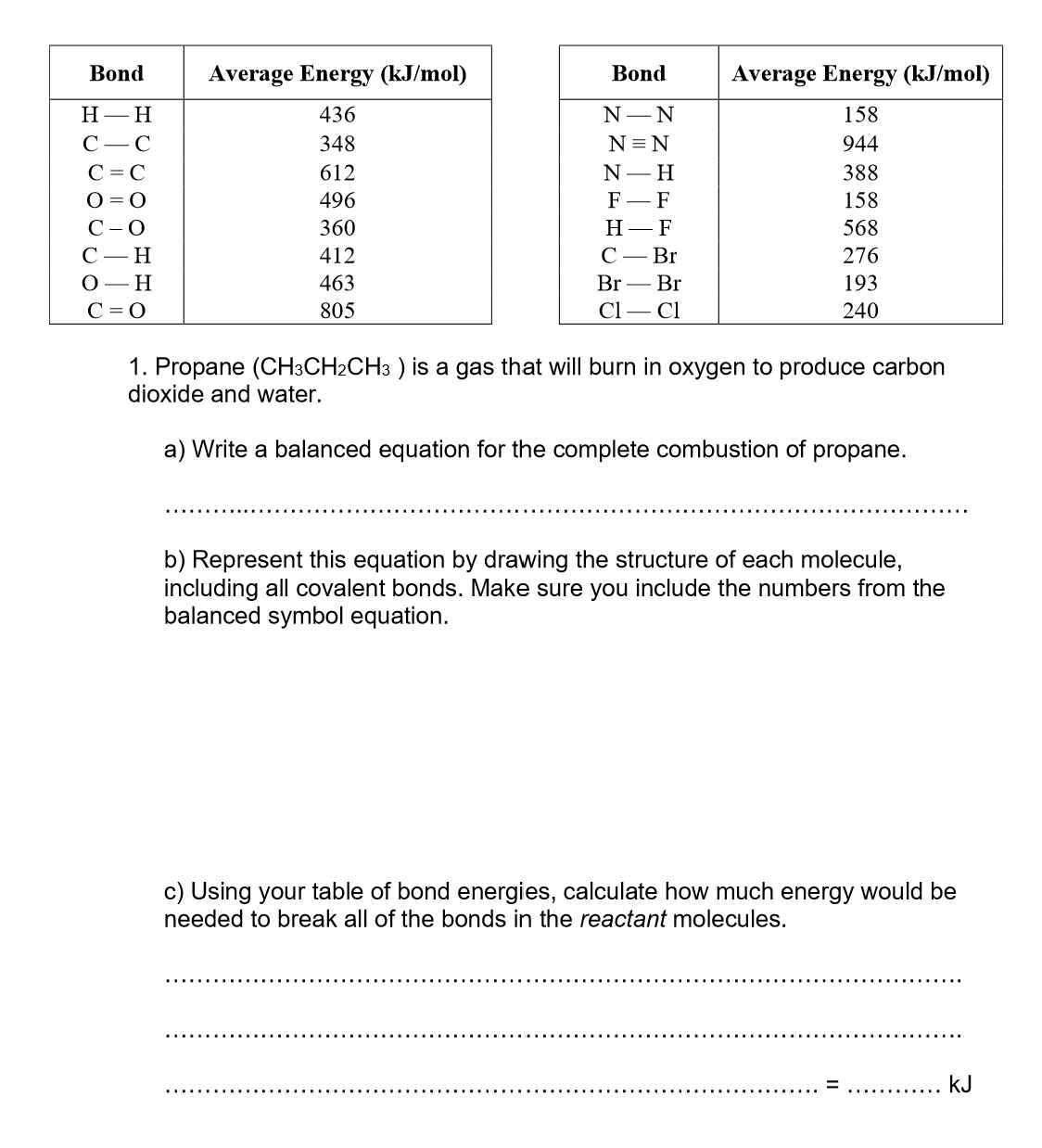
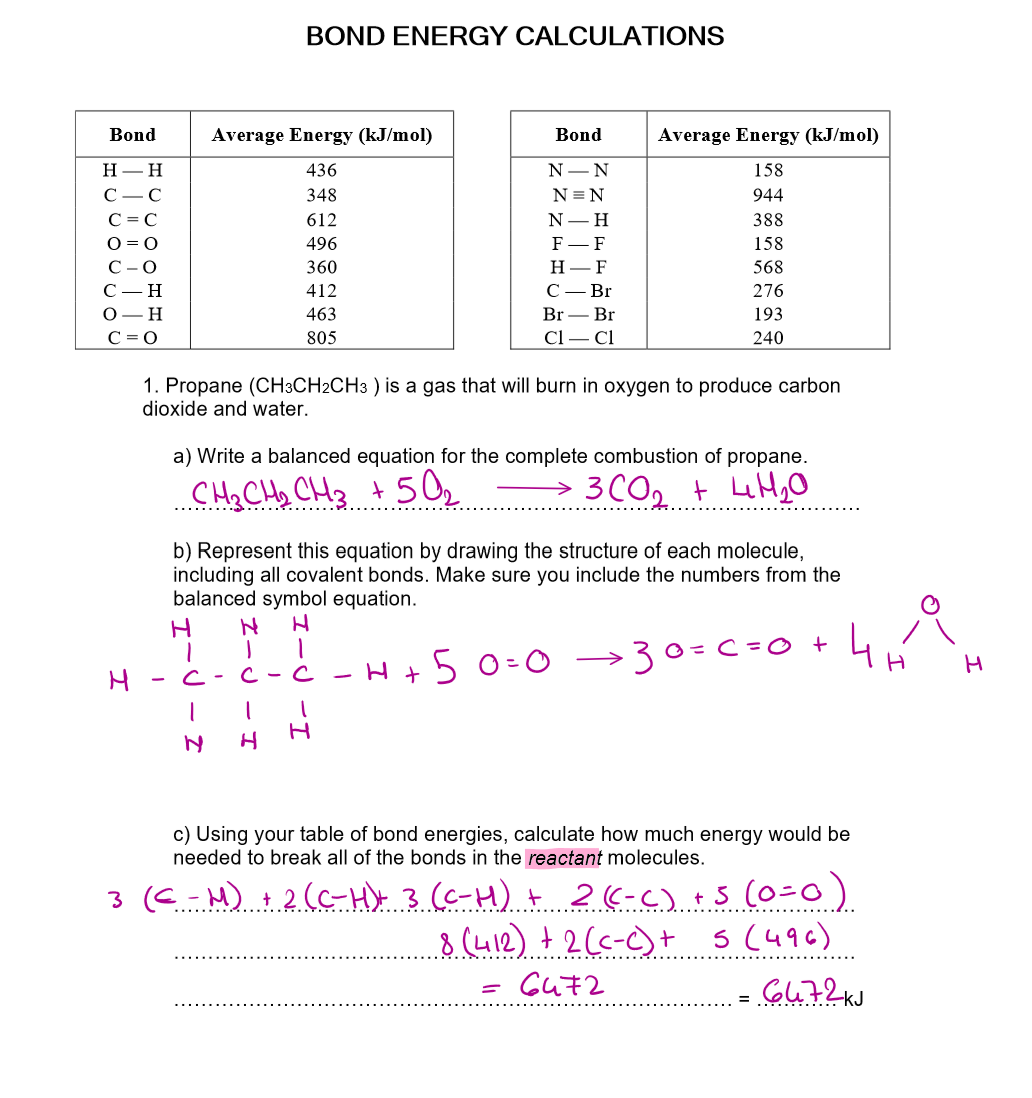
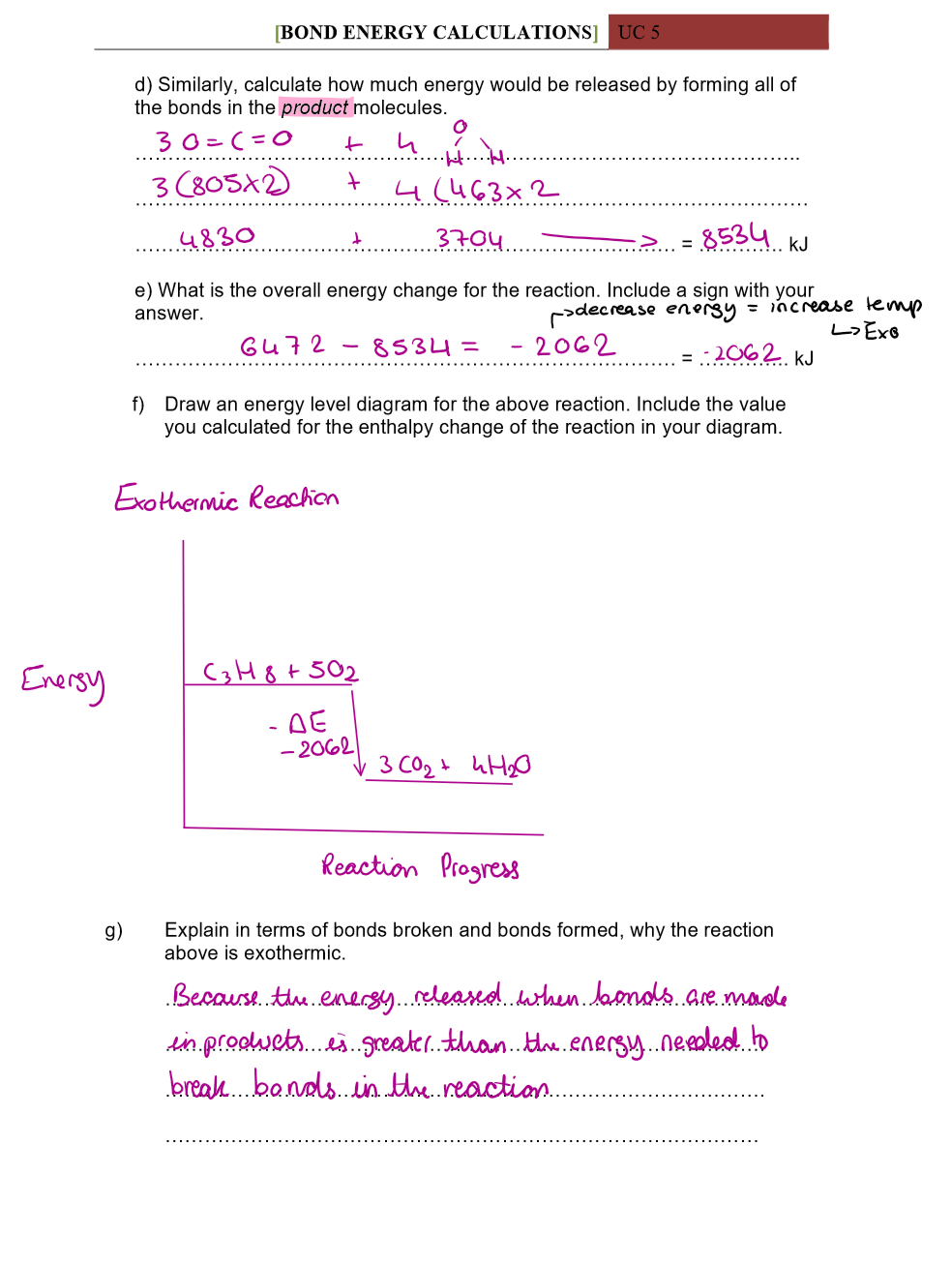
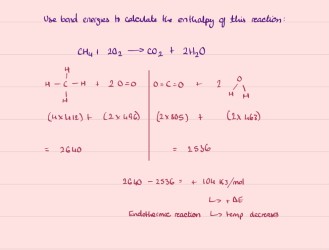
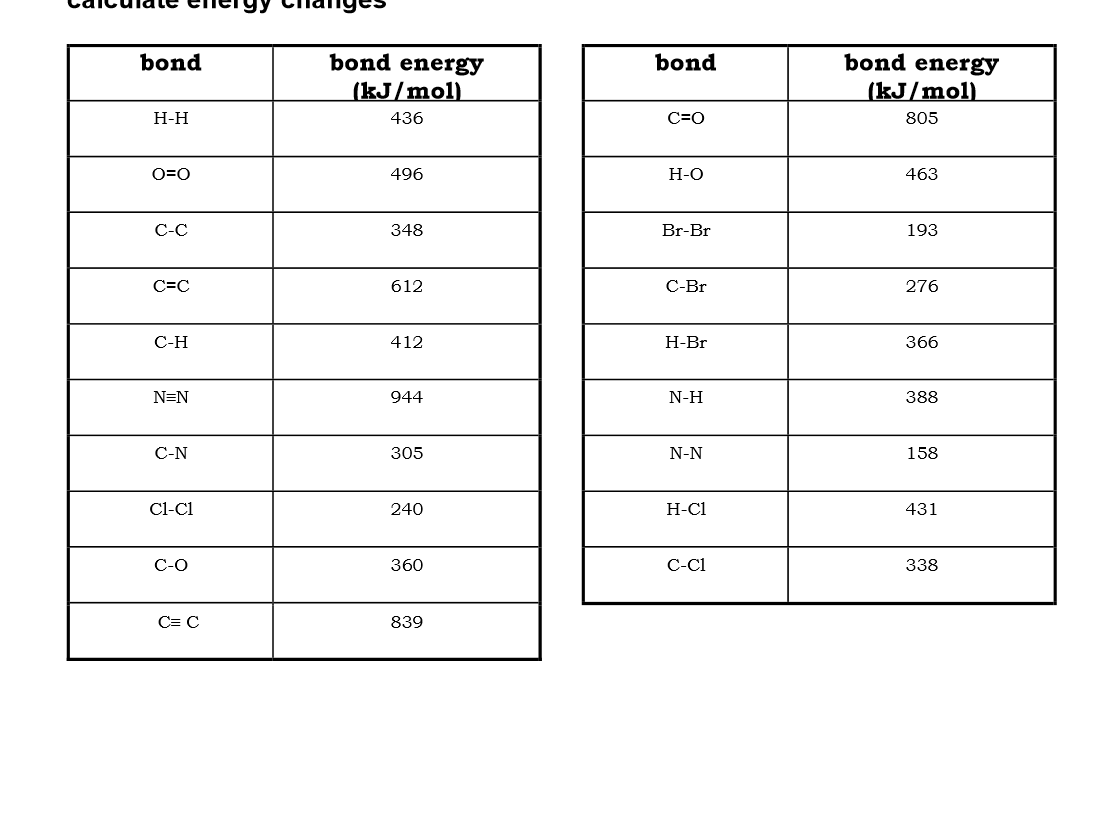
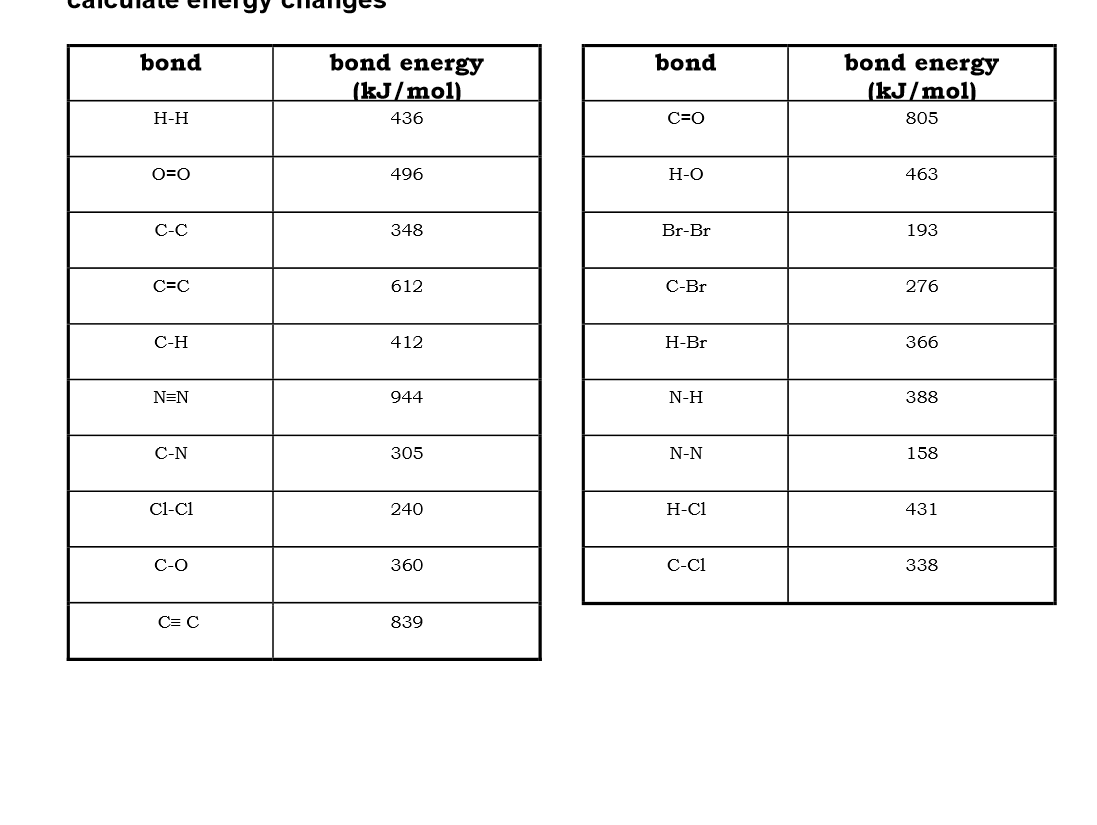
Use bond energies to calculate the enthalpy of this reaction:
CH4 + 2O2 → CO2 + 2H2O
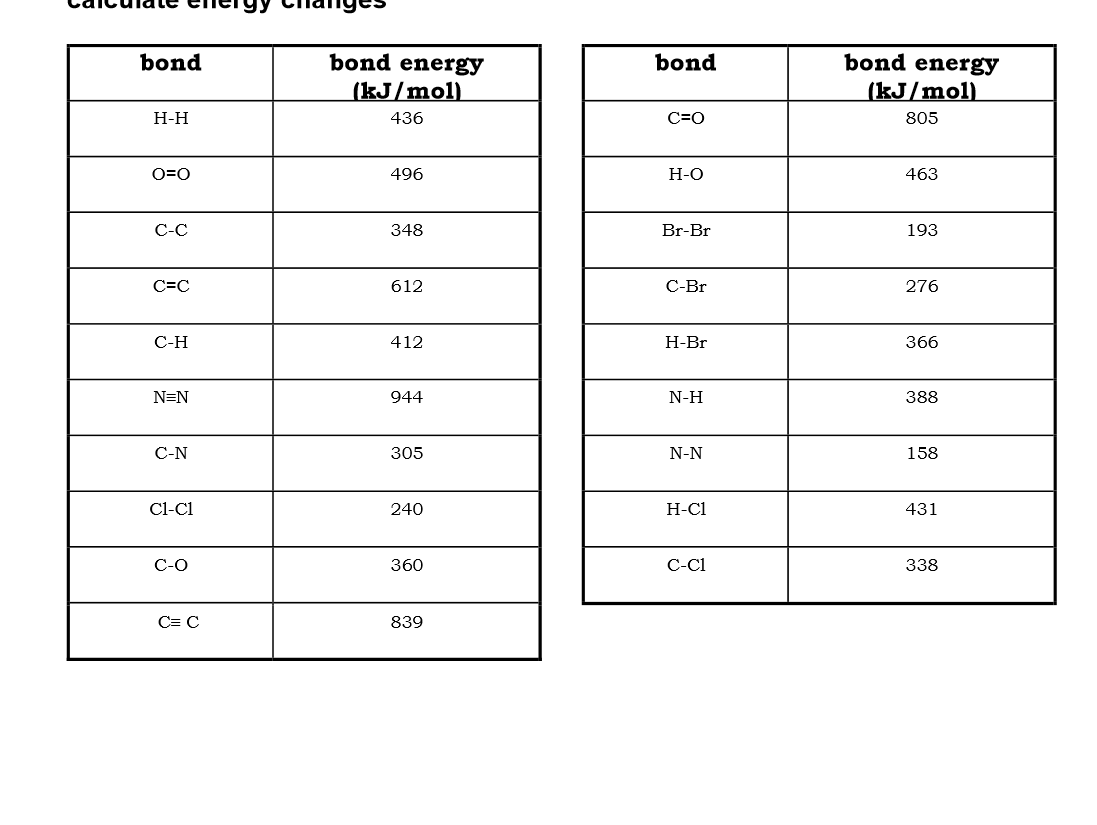
Use bond energies to calculate the enthalpy of this reaction:
2H2 + O2 → 2H2O
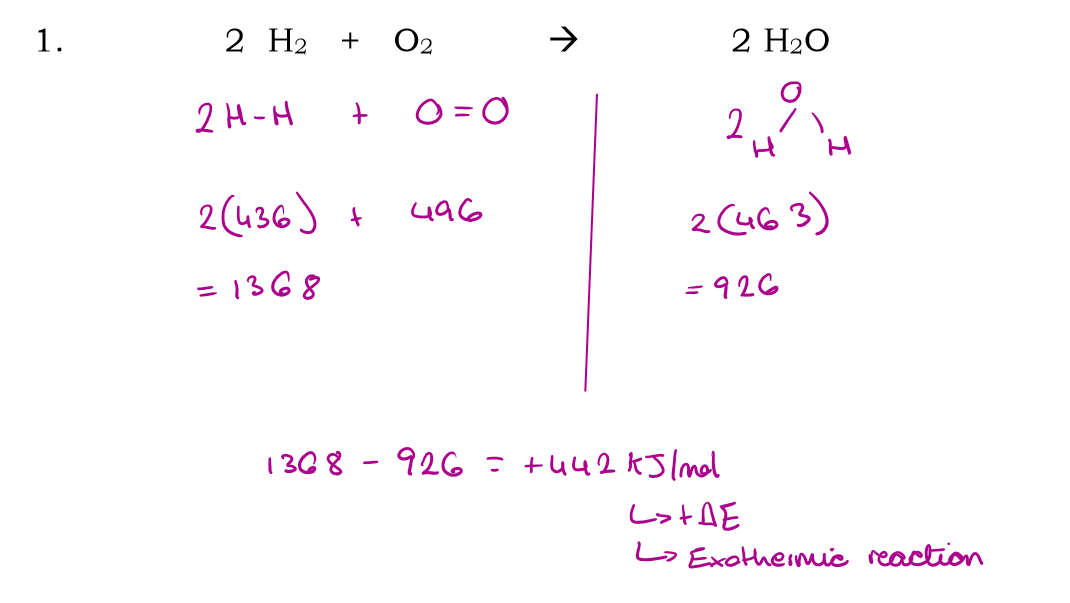
Use bond energies to calculate the enthalpy of this reaction:
N2 + 3H2 → 2NH3
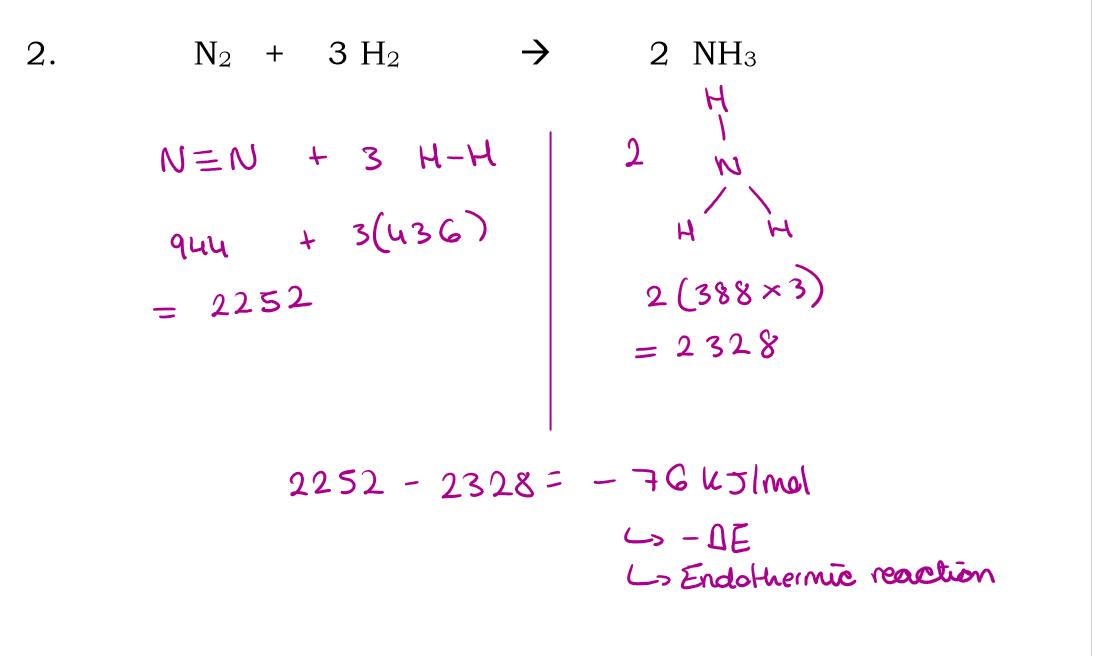
Use bond energies to calculate the enthalpy of this reaction:
CH4 (g) + 2H2O (g) → CO2 (g) + 4H2
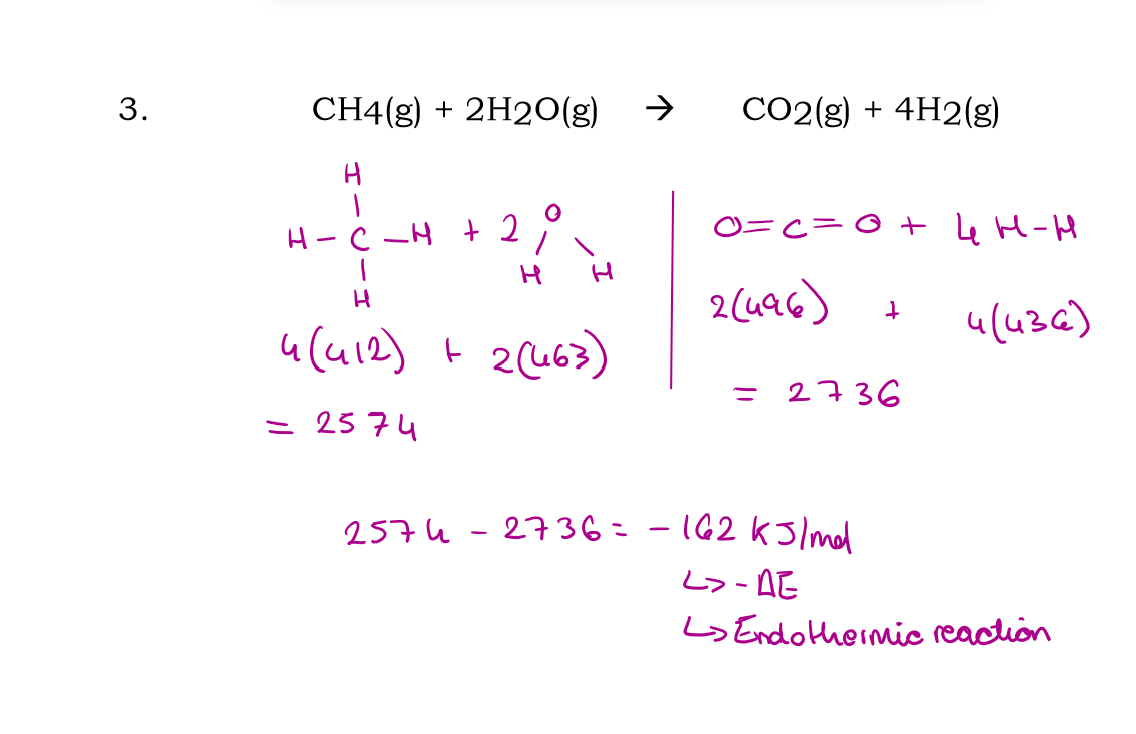
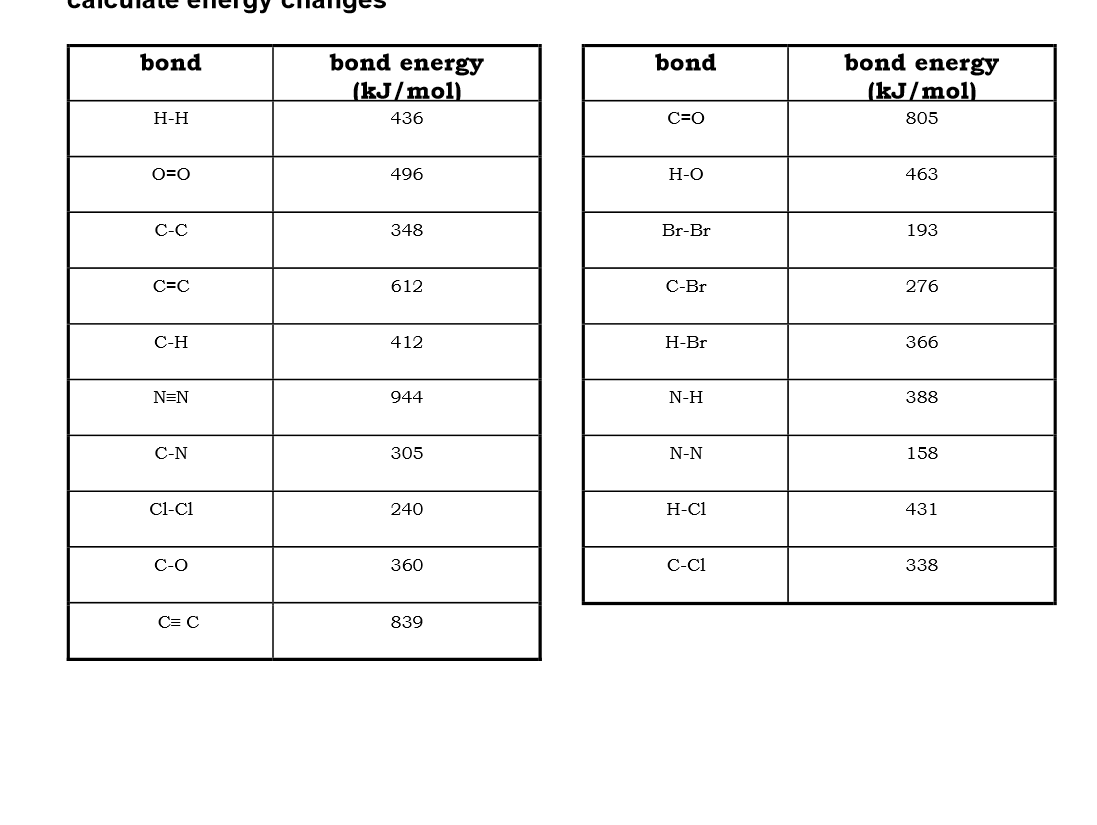
Use bond energies to calculate the enthalpy of this reaction:
Cl2 + 2HBr → 2HCl + Br2
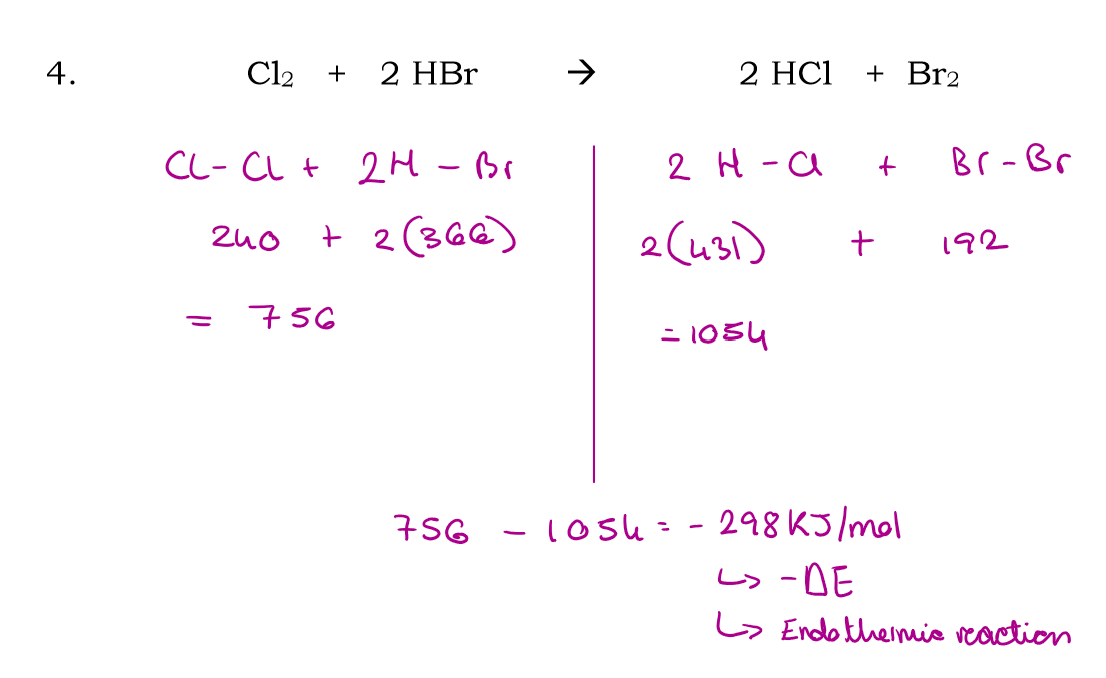
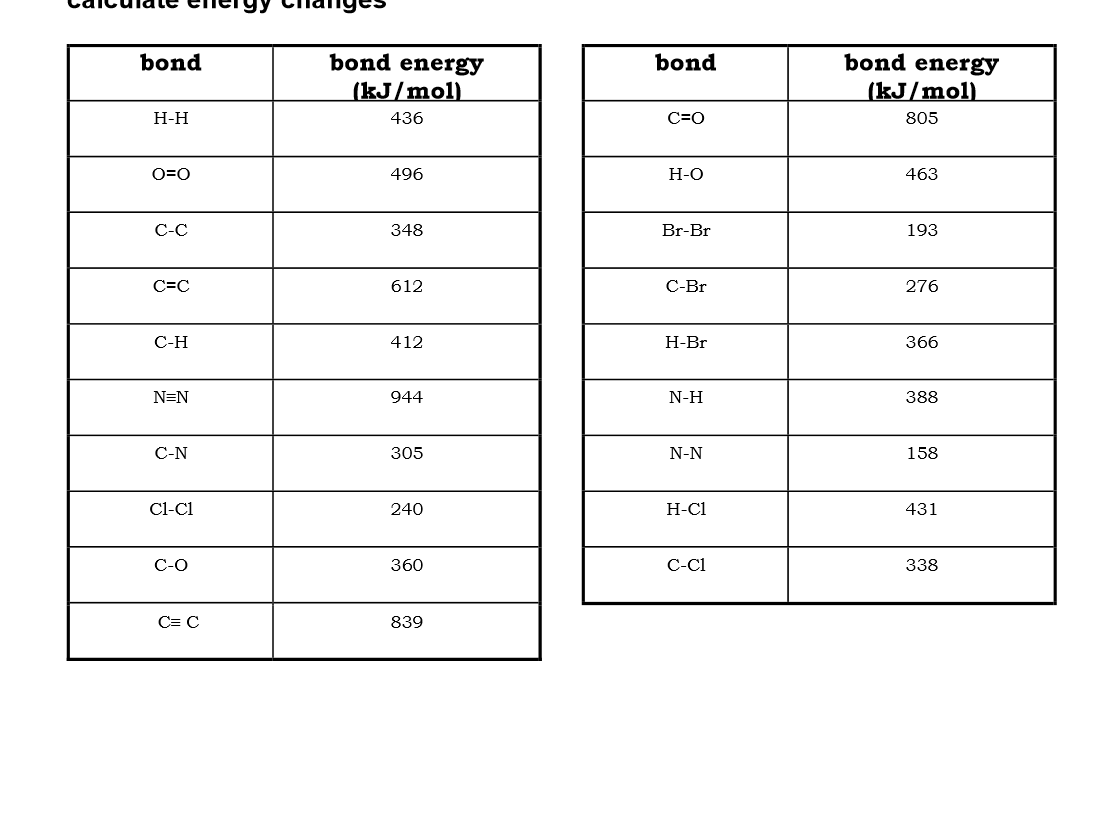
Use bond energies to calculate the enthalpy of this reaction:
CH2CH2 + Cl2 → CH2ClCH2Cl2
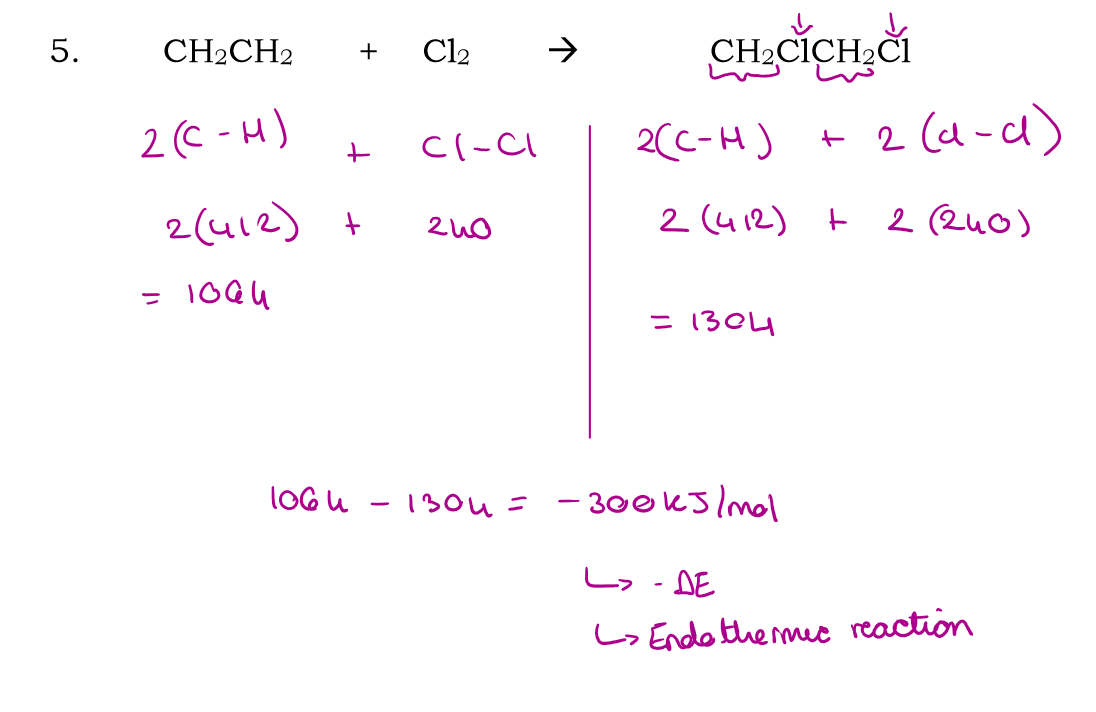
What is a reaction profile?
An energy level diagram tha shows the activation energy of a reaction
What are the 4 things you must include in your reaction profile diagram?
labels for the y -axis (energy) and the x- axis (reaction progress)
Names or formula of the reactants and products if known (if not jst reactants and products)
The energy change AE with a sign (- or +) and value (e.g 650) if known
The activatation energy , Ea
Explain why reactions are Exothermic in terms of bind energies
The energy released when bonds are made in products is greater than the energy needed to make bonds in reactants
Explain why reactions are endothermic
The energy released when bonds are made products is less than the energy needed to break bonds in the reaction
What is activation energy?
The minimum energy required for a collision to be successful and result in a reaction
Draw and label the reaction profile for an exothermic reaction
Draw and label the reaction profile for an endothermic reaction
Explain why the reaction between hydrogen and chlorine is exothermic (in terms of bond energies)
The energy released when bonds are made in products is greater than the energy needed to break bonds in the reaction
Explain why the reaction between hydrogen and oxygen is endothermic (in terms of bond energies)
Because the energy released when bonds are made in products is less than the energy needed to break bonds in the reaction
The reaction between hydrogen and chlorine is exothermic. Explain why this reaction releases energy to the surrounding
Because the energy released when bonds are made in the products is less than the energy needed to break bonds in the reactants